by Gertrud U. Rey

On October 2nd, 2023, the Nobel Assembly at Karolinska Institutet announced the award of the 2023 Nobel Prize in Physiology or Medicine to Katalin Karikó and Drew Weissman. The decision was based on a series of fundamental discoveries that led to the development of the COVID-19 messenger RNA (mRNA) vaccines.
Katalin Karikó had been interested in mRNA-based vaccines for many years, but she consistently faced a nagging problem. To induce an immune response to the protein encoded by the vaccine mRNA, the mRNA needs to remain stable until it is translated into protein. However, when exogenous mRNA is injected into an animal, the recipient’s innate immune system recognizes it as foreign and triggers an aggressive inflammatory response that degrades the mRNA. Karikó’s struggles in the lab were aggravated by lack of funding, because her grant proposals were repeatedly rejected, and by lack of support or understanding from her peers. In 1997, while working as an adjunct professor at the University of Pennsylvania, things were finally beginning to look up when she met Drew Weissman, a newly recruited immunologist who studied mRNA in the context of the immune system. After several chance encounters at a photocopying machine, the two struck up a conversation, recognized their common interest, and began a collaboration.
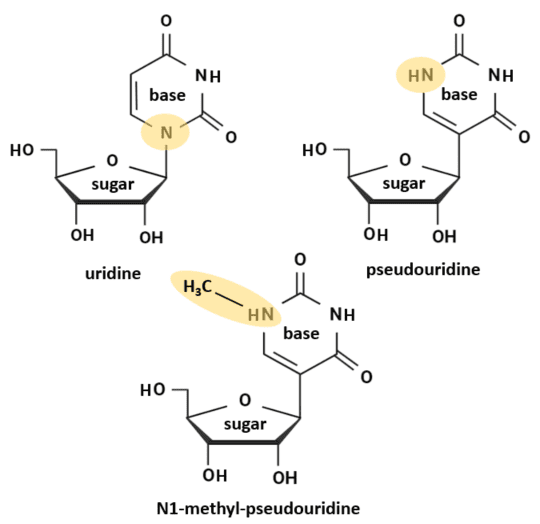
RNA is composed of building blocks called nucleosides that are attached to each other via a phosphate group. An RNA nucleoside has two main components: 1) a sugar molecule and, 2) one of four different types of nitrogen-containing bases – adenine (A), cytosine (C), guanine (G), or uracil (U). The names of the bases determine the names of the nucleosides into which they are incorporated – adenosine, cytidine, guanosine, and uridine (top left of image).
When Karikó and Weissman compared the properties of nucleosides derived from mammals to those derived from bacteria, they found that mammalian nucleosides are heavily modified through addition of methyl groups, substitution of sulfur atoms, rearrangement of the base relative to the sugar molecule, and other changes. In contrast, bacterial nucleosides contain few such modifications. They further noted that modified nucleosides had a suppressive effect on the innate immune system, which explained why endogenous (i.e., “self”) RNA usually does not induce inflammation compared to exogenous RNA from viruses or bacteria. Karikó and Weissman published their findings in a landmark paper in 2005, and they soon followed it up with two additional publications. A 2008 study showed that mRNAs containing a modified nucleoside called pseudouridine not only had particularly high immunosuppressive properties, but such RNAs were also more structurally stable and more easily translated into protein. Pseudouridine is a variation of uridine in which the uracil base is rotated to attach to its sugar molecule via a carbon rather than a nitrogen atom (top right of image). A publication in 2010 showed that the higher rate of translation of pseudouridine-containing mRNAs is attributable to reduced activation of RNA-dependent protein kinase, a mammalian enzyme that normally represses the translation of pathogen-derived mRNAs. Collectively, these findings suggested that mRNAs containing modified nucleosides might be stable enough to be used as vaccines.
Karikó and Weissman’s discoveries provided an important basis for subsequent vaccine development. Addition of a methyl group to one of the nitrogen atoms in pseudouridine produced N1-methyl-pseudouridine (bottom of image), and mRNAs that contained this nucleoside were even more immunosuppressive and more easily translated. Encapsulation of mRNAs into lipid nanoparticles enhanced both the stability of the mRNAs and their delivery into cells. Cholesterol and polyethylene glycol molecules embedded in the nanoparticle membrane improved fusion of the particle with target cell membranes and increased vaccine efficacy by slowing down its degradation.
Clinical trial recruitment for an mRNA vaccine against rabies began in 2016, and additional trials for vaccines against influenza virus, cytomegalovirus, Zika virus, and others soon followed. By the time the COVID-19 pandemic began, mRNA vaccines had already been found to be both safe and effective in tens of thousands of individuals. Because the SARS-CoV-2 spike protein was predicted to be highly immunogenic based on previous studies on other coronaviruses, and because the genetic sequence of the virus was determined early during the pandemic, it was relatively easy to insert the spike protein-encoding gene into the already existing mRNA vaccine framework. It is therefore not surprising that clinical trials for COVID-19 vaccines could begin within months of the start of the pandemic.
Karikó and Weissman’s foresight and persistence have saved millions of lives. I hope that those who control the funding of biomedical research will remember this story and keep an open mind to ideas that seem outside of the box.
[The 2005 paper was discussed in detail on TWiV 860 and the award of the Nobel prize was discussed on TWiV 1051.]

Amazing article, very well explained and straightforward. My best regards to the author.
Why can’t we get the RNA needed from RNA genomes rather than converting DNA genome segments into mRNA (On The Origin Of A Genetic Constant – 7)?
The reason I ask is the The Central Dogma of microbiology is not atomic (On The Origin Of A Genetic Constant – 5).
I am glad that Katalin Karikó and Drew Weissman are NOT victims of Stigler’s law of eponymy. I expect to be a victim of it RE the “~(32/35/25/6) genetic constant” discovery (it is useful for detecting DNA contamination).
Sorry about the bad link above. see here
This recognition by the Nobel Assembly at Karolinska Institutet reflects the profound impact of Karikó and Weissman’s contributions to the field of mRNA research and vaccine development.