Centers for Disease Control and Prevention have determined that some adults have serum cross-reactive antibodies to the new influenza H1N1 virus. One of the techniques used to reach this conclusion is the hemagglutination inhibition (HI) assay. How does this assay work?
To understand the HI assay, we must discuss the hemagglutination assay. Influenza virus particles have an envelope protein called the hemagglutinin, or HA, which binds to sialic acid receptors on cells. The virus will also bind to erythrocytes (red blood cells), causing the formation of a lattice. This property is called hemagglutination, and is the basis of a rapid assay to determine levels of influenza virus present in a sample. To conduct the assay, two-fold serial dilutions of a virus are prepared, mixed with a specific amount of red blood cells, and added to the wells of a plastic tray. The red blood cells that are not bound by influenza virus sink to the bottom of a well and form a button. The red blood cells that are attached to virus particles form a lattice that coats the well. The assay can be performed within 30 minutes, and is therefore a quick indicator of the relative quantities of virus particles.
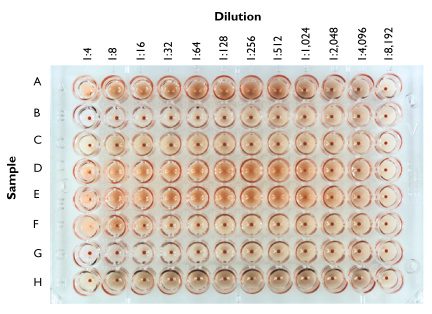

In the figure above, two-fold dilutions of samples of different influenza viruses (A – H) were prepared, mixed with chicken red blood cells, and added to the wells of a 96-well plate. After 30 minutes the wells were photographed. Sample A causes hemagglutination up to the 1:256 dilution; therefore the HA titer of this virus stock is 256. The sample in row B contains no detectable virus, while that in row D has an HA titer of 512.
The HA assay can be easily modified to determine the level of antibodies to influenza virus present in serum samples. In the CDC study cited below, the authors wished to determine whether stored serum samples contained antibodies to the new influenza H1N1 strain. First they obtained a preparation of one of the new influenza viruses, specifically A/California/04/2009 and determined its HA titer by the method described above. They added a fixed amount of virus to every well of a 96-well plate, equivalent to 32 – 64 HA units. Then they prepared two-fold dilutions of each serum to be tested, and added each dilution series along a row of wells. Finally, they added red blood cells and incubated for 30 minutes.
The basis of the HI assay is that antibodies to influenza virus will prevent attachment of the virus to red blood cells. Therefore hemagglutination is inhibited when antibodies are present. The highest dilution of serum that prevents hemagglutination is called the HI titer of the serum. If the serum contains no antibodies that react with the new H1N1 strain, then hemagglutination will be observed in all wells. Likewise, if antibodies to the virus are present, hemagglutination will not be observed until the antibodies are sufficiently diluted.
The CDC report contains the statement “…serum HI antibody titers of 40 are associated with at least a 50% reduction in risk for influenza infection or disease in populations”. A serum HI antibody titer of 40 means that at a dilution of 1:40, but not higher, the serum blocked hemagglutination. By determining HI titers and comparing them with influenza attack rates in populations, it is possible to calculate the significance of the HI antibody titer with respect to susceptibility to influenza virus infection. When used in this manner, the HI assay is a powerful epidemiological tool.
J Katz, PhD, K Hancock, PhD, V Veguilla, MPH, W Zhong, PhD, XH Lu, MD, H Sun, MD, E Butler, MPH, L Dong, MD, PhD, F Liu, MD, PhD, ZN Li, MD, PhD, J DeVos, MPH, P Gargiullo, PhD, N Cox, PhD (2009). Serum Cross-Reactive Antibody Response to a Novel Influenza A (H1N1) Virus After Vaccination with Seasonal Influenza Vaccine Morbid. Mortal. Weekly Rep., 58 (19), 521-524
Potter, CW, & Oxford, JS (1979). Determinants of immunity to influenza infection in man. Br Med Bull, 35, 69-75

why we perform HI test…..commercial benefits , diagnostic benefits, etc
HI assay is done to determine the level of specific viral antibodies
in serum. In humans, this information is useful for many reasons,
including determining the extent of infection of a population with a
particular virus strain, or to test vaccines. In animal models
measurement of viral antibodies provides information on experimental
virus infection or vaccine efficacy.
Great information.
I was wondering what the difference is between seroprotection (so in this case a HA inhibition of at least 1:40) and seroconversion? Can seroconversion values be used the measure the efficacy of influenza vaccination?
Also, I went through your posts on adaptive immunity. Is it possible to measure the effectiveness of the vaccine not just using humoral response but also T-cell activation? If so, how can this be done.
I also have one last question. Do you think the influenza vaccine (which is currently annually recommended in COPD patients) would be less effective in COPD patients because they may have a weakened immune system?
Thank you very much
Many thanks for this clear explanation of the HI assay.
I wonder if you could explain why in some cases the HI assay results show a higher heterologous titer than homologous, or refer me to any recent papers on the subject?
Thank you for the great information posted! I can't imagine how much time this demands from you…
I have always wondered if infection and vaccination produce the same kind of antibody immunity (in terms of amounts, isotypes, receptor-binding capacity, etc). Also, if they are different, can this be an issue targeted to achieve better protection and/or increase cross-reactivity of Abs produced?
Many thanks
The following lab can do influenza HA titers as well as the hemagglutination inhibition test and other flu testing services for non-clinical purposes. http://www.virapur.com
The responses to natural infection and immunization with inactivated
vaccine are quite different. The latter has little viral RNA and
internal proteins such as NP and therefore doesn't induce good
inflammation. The response is likely less cross-reactive as a
consequence. Flumist should be better but hasn't been used long enough
to know.
The following lab can do influenza HA titers as well as the hemagglutination inhibition test and other flu testing services for non-clinical purposes. http://www.virapur.com
The responses to natural infection and immunization with inactivated
vaccine are quite different. The latter has little viral RNA and
internal proteins such as NP and therefore doesn't induce good
inflammation. The response is likely less cross-reactive as a
consequence. Flumist should be better but hasn't been used long enough
to know.
Hi profvrr,
1st congratulation for your blog… very interesting!
2nd I have a question regarding the HI assay.
I'm currently analysing serum with the HI and MN (microneutralisation) assay… I was wondering what kind of AB I would detect with the 2 tests. Both tests detects AB raised against the receptor binding site of the HA but I thought that the MN assay would cover a broader range of AB (against other flu AG such as the NA, NP etc) and other parts of the HA (e.g. AB against the HA cleavage site…). An expert told me the opposite so that I'm a bit lost… What's your opinion about that …
Thanks for you reply
Hello!!!
WOW this blog is incredibly helpful!!
I'm performing this assay and you mentioned that a fixed amount of HA (virus/vaccine) is used. The protocol I'm using does 4HAU and pretty much every paper I run into does the same. That fixed standard amount is because 40 HI titer is associated with 50% reduction of “Flu” risk??
Thanks.
hi, i want an animation movie about HI test, would you plz help me to find it out.
Did you ever have problem about your HA titer(virus titer) in your HI assay???
I get the virus titer from HA 1:128 ,Iwant to dilute to 4HA in HI then i dilute virus in dilution 1:32.The virus back titration with HI assay don’t be 4HA….sometime less than but sometime more than….I don’t know how to solve this problem.
Sounds like you have pipetting problems. Make sure they are properly
calibrated – if you are using pipetmen.
I have a question somehow related to this topic: As far as I know, Hemagglutinin was characterized and subtyped using double immunodiffusion. The antibodies used for that came from some selected stems. Antigenic drift cause minor chances in the hemagglutinin and therefore cause to “escape” the immunreaction (therefore also vaccines are updated from time to time). So if those antibodies don’t longer work due to altered binding sites (I have tried to read about the binding sites but it’s way to long to read through the papers dealing with it for me lacking in time and I had the impression I wouldn’t find my answer there anyway), how “minor” are the changes and how can it be on the other hand, that antibodies work quite fine to determine it as specific subtype. In other words: How can we have antibodies in test sera that widely cross-react on the one hand (which surely have been conservated from some animal somewhen) but on the other hand we see humans having no cross-immunity to different strains.
I mean, this is so contradictory to me – hope you can bring some light to this and thank you already in advance!
The antibodies in test sera do not widely cross-react – you must use a
panel of sera, each against a specific viral subtype. If your viral
isolate does not react with any of the antisera, you may conclude that
it has drifted.
Thank you very much for the quick reply! I understand that logic – no reaction may be due to a drift (or even just found a new subtype…). But my question was rather for the opposite case:Â A drifted Influenzavirus can appear new to the human immunsytem when having no pre-existing antibodies (or antibodies against other Influenzaviruses don’t cross-react) – but how can it be, that it still can be subtyped e.g. as H1 (talking general, not for H1N1v). That is my main question, how can we already have test-sera against something “new”. Sure, logic thinking leads me to some assumptions, but it is hard to find out how this subtyping is done and I’d really would like to be sure, for personal interest. Thinking logically, I’d assume that the test sera must have a broader range of antibodies than those produced by humans. Is this just the point? That mixing together antibodies against hundreds of diefferent H1 just increases the possibility of a positive reaction? Thank you!!
Hi..
     Why is it that we use an acidic pH of 6.2 to 6.4 for arbo virus HA tests? I mean dats not wat happens in vivo right?
Â
Pingback: ANKYLOSING SPONDYLITIS RESEARCH | TNF Alert
Dear Dr. Vincent
I have some problems doing the HA titer of influenza using the basic normal method, Although I am having a high plaque titer of influenza about 1×10^8 PFU/ml I can not get any activity for the HA assay. Is there a relationship between virus plaques and HA titer. I have also another questions, do you know why sometimes while doing the HA assay we get the blood spot at early dilution 1:2 and 1:4 and then it disappears on dilution
haredy
just finished doing HA test for TCF cultured influenza virus. Virus did not show haemagglutination although same sample cultured in egg is showing HA titre of 1:512. Any idea why this could have happened?
Pingback: How good is the influenza vaccine?
hello prof, I have some question regarding HI assay. Last week is my first time doing HI assay for my practical class. Could you mind to share some disadvantages of HI assay? I still wondering that for unknown sample, and the HI titre is high, how could we know that the high production of antibodies is due to vaccination or infection (secondary or past)?
Pingback: Should we fear avian H5N1 influenza?
How would you report HAI results? Is it log2 or log3?
Log2.
Why do we chose 4HA for HI test?
I believe the first paragraph of this article may be incorrect. Are you not suppose to add a specific amount of influenza virus to each well and then serially dilute the sample blood? You want to dilute the antibodies in the blood sample and see how they respond to a set amount of influenza virus.
Yes, most of the description is incorrect. The figure is also incorrectly labelled, the dilutions are listed in reverse order. The lower dilutions should all have red blood cell “buttons” and only at higher dilutions should the buttons disappear.
You are both confusing the hemagglutination assy the Dr.Vincent is talking about  with the hemagglutination INHIBITION assay.
You guys are referring to the latter.
Pingback: More evidence for mild influenza H5N1 infections
Right, but in vitro, these are the pHs that have been standarized
Rafael
carrying out the assay with human blood will it give the same result
I have a problem with preparation of RBC, we use 1% RBC for AI test, but the tear of RBC has no standart always too small, and i always found white sediment in the RBC after washing with PBS, i use alsever for anti koagulant, do you have any idea about my problems?
Hi ,
hope this blog is still active…
Does some one know when it is better to use U-shape or V-shape plates for Influenza HI?
Does some one have any good picture that shows was is considered positive and what is negative result?
Should  a V-shape plate be tilted to get a relaible result?Â
Many Thanks
Nice to see the useful questions and answers. It seems so much more quiet about viruses in 2012.
hi, may i ask clear protocol for erythrocyte purification? we are planning to use rooster’s blood. is there special treatment for this? I hope i can have your replies, Im such a neonate in this field. Thank you.
you should dilute to 8HAU/50 ul (4HAU/25 ul) because the HA titer is usualy given in HAU/50 ul units, so you have 128HAU/50 ul.
Pingback: Flu vaccine shown to not be effective in 18-49 year olds « Truth… In a Nutshell
Results will be the same, but will take longer to show. The only reason chicken blood was used was because they have nucleus in it, hence heavier, allowing them to settle to the bottom of the wells faster so results can be observed faster.
Would free HA in the sample also cause the RBC to agglutinate? I am asking because I am working with influenza VLP production, and I am wondering if I could get a false positive from free HA in my samples that are not incorporated into the VLPs?
Pingback: TWiV 223: EEEV and the serpent
The stars of your pool include the comments additionally, the pictures can be secondary. Don’t incorporate photos utilizing only congrats and also praise.
In fact, we didn’t even be able to those containers until the center of the fall.
Our admins have a very good sharp eye in addition to sharper senses – and also our Perfect Comments network enjoys an ideal read. Come play around!
Very nice explanation of HA
We have recently developed a module to analyze HA plates automatically based on computer vision.
Can be seen here
http://www.scirobotics.com/products/hemagglutination-analyzer
Pingback: Inefficient influenza H7N9 virus aerosol transmission among ferrets
Pingback: Incidence of asymptomatic human influenza A(H5N1) virus infection
is HI assay only done for viruses??