by Gertrud U. Rey
This post was written in honor of Virus Appreciation Day, which occurs annually on October 3.

Public awareness of human immunodeficiency virus (HIV) began in the early 1980s when separate clusters of infected individuals were identified in Los Angeles, San Francisco, and New York. These individuals all shared a group of symptoms later named “acquired immunodeficiency syndrome” (AIDS) – a disease state that is characterized by a profound susceptibility to infections and cancers that don’t typically affect people with a competent immune system. Even though the quest for a vaccine to prevent and/or cure HIV infection began immediately, nearly half a century later we still don’t have one.
There are multiple hurdles that have prevented progress in vaccine development in this field. The most glaring obstacle is the fact that HIV integrates into the host genome and becomes part of that person’s DNA for life. After entering a host cell, the viral-encoded reverse transcriptase enzyme converts the HIV RNA genome into DNA, which is then inserted into the host genome by another viral enzyme named integrase. Each new round of replication of the host’s DNA also produces new copies of the inserted viral genome. Newly replicated HIV DNA is transcribed into RNA, which gets packaged into newly synthesized viral particles that are assembled with the help of a viral enzyme named protease. Taking this sequence of events into consideration with the caveat that most vaccines prevent disease but not infection, any potential HIV vaccine would probably exert its function too late. In other words, a vaccine that induces an immune reaction to a virus that has already infected the host might not be as effective if the virus has already integrated into the genome.
After infection and integration, HIV persists in a pool of cells collectively known as the HIV reservoir. These cells consist primarily of a certain subtype of T cells (i.e., T helper cells, discussed below), but can also include macrophages, microglia, and astrocytes. HIV reservoir cells are capable of increasing in number, and it has proven extremely difficult to identify and eliminate such pools of cells. Even the small group of HIV-infected individuals known as “elite controllers” who are able to maintain suppressed viral levels without antiviral medication retain a low frequency of intact integrated HIV DNA copies in their peripheral T helper cells.
An additional hurdle in HIV vaccine development has to do with the high rate of mutations that occur during viral replication. Unlike other enzymes of its type, HIV reverse transcriptase does not have a proof-reading mechanism, meaning that it introduces multiple errors into each new strand of DNA during each round of replication. Furthermore, each viral particle contains two copies of single-stranded RNA, and reverse transcriptase often switches back and forth between the two strands, thus producing DNA copies that are recombinants of the original two RNA templates. Combined with the rapid rate of replication of HIV, these factors lead to the generation of infinite numbers of viral variants in any given infected person. To demonstrate, consider the following numbers:
- The rate of mutation of HIV reverse transcriptase is about 0.000034 mutations per nucleotide per replication cycle.
- The HIV genome is about 10,000 nucleotides in length.
- An infected person produces about 100,000,000,000 new virions each day.
It then follows that an infected individual can generate about 34 billion viral variants in a single day. Designing a vaccine that induces broadly neutralizing antibodies and/or T cells to so many different variants is daunting.
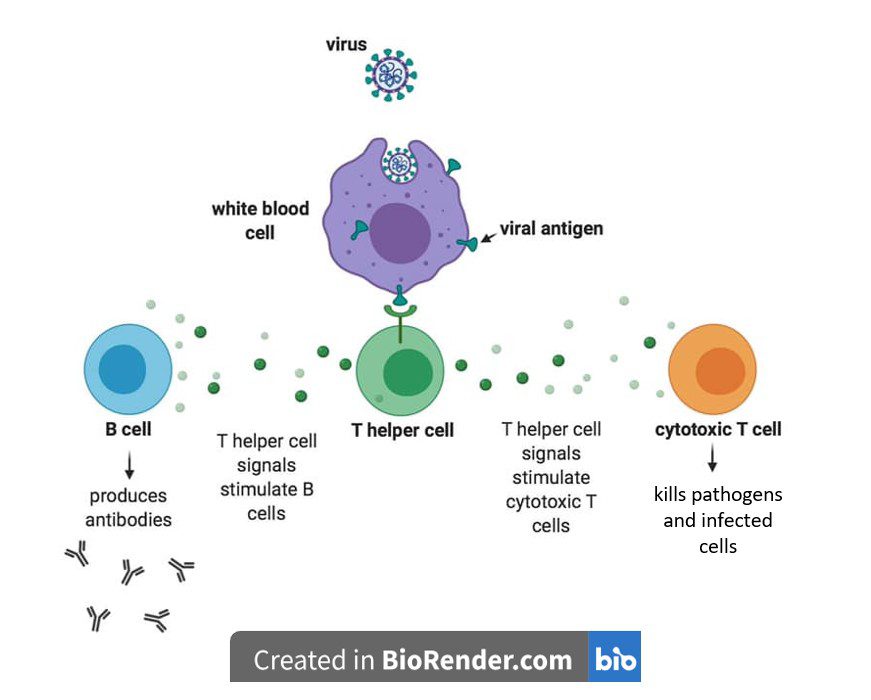
The immunodeficiency aspect of HIV disease stems mainly from the fact that HIV infects and kills the cells that are central to the adaptive immune response. Although HIV also infects macrophages and dendritic cells, as the infection progresses, the virus primarily targets T cells that harbor CD4, a cell surface protein that aids HIV entry into cells. These CD4+ T cells, also known as T helper cells, are essential for activating the adaptive immune response, because they produce signaling molecules that stimulate B cells to produce antibodies and cytotoxic T cells to kill pathogens and infected cells (see image). As more and more T helper cells are infected and killed, they progressively decline in number. Because there are insufficient T helper cells, B cells are not stimulated to make antibodies, and cytotoxic T cells are not activated to attack invading pathogens – a situation that leads to increasingly compromised immunity.
The development of anti-retroviral therapy (ART) in the mid-1990s led to a major turning point in the HIV pandemic because it marked the end of HIV infection as an inevitably accelerated death sentence. ART consists of a combination of drugs that inhibit the function of various factors involved in the viral replication cycle. These factors include proteins that facilitate viral binding and cell entry, and enzymes like reverse transcriptase, integrase, and protease. When properly adhered to, ART reduces the amount of HIV in the body to an undetectable level, thereby decreasing the risk of transmission to others while greatly increasing the life expectancy of the patient.
In the meantime, efforts are still underway in the field of HIV vaccine research, particularly with the advent of artificial intelligence (AI), which has provided a variety of new tools that aid in vaccine design. AI-assisted predictive modeling can be used for various purposes, including clarifying the mutational pressures of HIV, estimating immune responsiveness to various drug delivery systems, identifying broadly neutralizing antibodies, and managing the complex workflow of clinical trials. However, considering the numerous barriers described above, which are not all-encompassing, any promise of an HIV vaccine should be perceived with caution and a healthy dose of skepticism.
[For a video version of this post, check out Catch This Episode 65.]

Here is my testimony on how to get cured from HSV1&2. I got diagnose of HSV-2 and I have been taking pills to prevent an outbreak. I never stop searching for a cure because I strongly believe that there is something somewhere that can get rid of it completely and in February this year, I ran through some comments on a blog about Dr Osato herbal cure and a lot of people commented about him having the herbs that can get rid of herpes completely. I was excited and I contacted Dr Osato and ordered the cure for myself and he sent it to me through UPS and gave me instructions on how to take it which I rightly followed and behold I went for checkup after two weeks of taking the herbs and my result shows NEGATIVE. My doctor confirmed with me that I am totally clear from HSV-2. You can as well contact Dr Osato to get the herbal cure from him. His website is https://osatoherbalcure.wordpress.com
I recommend more research in use of Honey with other medications for treatment of herpes virus. I stumbled on NZE NJOKU HERBAL HOME on google when I was searching for alternative remedy for genital herpes and after treatment with honey together with other medications I have never seen an outbreak. Not even from stress