In our quest to stop the COVID-19 pandemic by vaccination, we have been myopically focussed on inducing antibodies against the spike protein. As variants of SARS-CoV-2 have emerged that reduce the ability of such antibodies to block infection, concern has arisen that we will not be able to halt the disease. Such concerns appear to ignore the other important arm of the adaptive immune response: T cells.
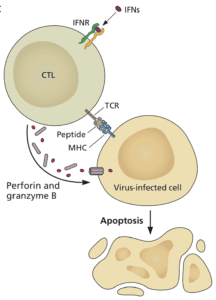
Anti-viral antibodies can prevent infection of cells, but when antibody titers are low – years after infection or vaccination – some cells will inevitably be infected. In this case, T cells come to the rescue. Cytotoxic T cells can sense that a cell is infected and kill it (illustrated). T cells sense infected cells by virtue of viral peptides that are presented by major histocompatibility molecules on the plasma membrane. Such T cell peptides may be produced from nearly any viral protein. In contrast, only certain viral proteins, like the spike of SARS-CoV-2, can give rise to antibodies that block infection.
The T cell response to SARS-CoV-2 infection has been largely ignored for the past year. Certainly, some laboratories have studied T cell responses in patients, and the vaccine makers have dutifully included them along with assays for neutralizing antibodies. But the dialogue has never included T cells as important for resolving disease – but they are for most viral infections. Because T cells can kill virus infected cells, they can help prevent disease and end the infection.
The recent finding that amino acid changes in the spike protein of SARS-CoV-2 variants of concern do not impact T cell reactivity is very good news. In this study, the authors synthesized short peptides covering the entire proteome of multiple SARS-CoV-2 isolates, including the original Wuhan strain, and variants B.1.1.7, B.1.351, P.1, and CAL.20C. They found little difference in the ability of T cells from either convalescent or vaccinated patients to recognize peptides from these viruses. This result means that the amino acid changes in the variants are not likely to impact the ability of T cells to clear infection.
This observation explains why some COVID-19 vaccines have effectively prevented hospitalization and death even in regions where variants circulate widely. In some cases the ability of sera from vaccinated individuals have reduced ability to neutralize infection with some variants. Nevertheless, the vaccines prevent severe COVID-19 and death because T cells can still recognize variant virus-infected cells and clear them.
It’s highly unlikely that vaccination will prevent infection with SARS-CoV-2. Antibody levels rapidly decline after infection or vaccination, especially in the respiratory mucosa. When a virus enters the nasopharynx of an immune individual, it will encounter little antibody opposition and will initiate an infection. However memory B and T cells will spring into action and within a few days produce virus-specific antibodies and T cells. The antibodies will limit infection while the T cells will clear the virus-infected cells. The result is a mild or asymptomatic infection that likely is not transmitted to others.
The recent observations that vaccination appears to prevent asymptomatic infections is a red herring. These studies are being done soon after vaccination when antibody levels in serum and mucosa are high. If these studies were done a year after immunization, the results would be quite different.
Now imagine that you are fully vaccinated and become infected with a SARS-CoV-2 variant. The virus may begin to reproduce rather well in the nasopharynx even in the face of a memory response, because the antibodies are just not good enough to block infection. T cells to the rescue: the T cell epitopes on the surface of the infected cells are readily recognized because they are mainly the same in the variants as in the ancestral strain of SARS-CoV-2. You may have a mild infection but you will not be hospitalized or die. Isn’t that the goal of vaccination?
Why don’t T cell epitopes change as do B cell (antibody) epitopes? A B cell epitope is the same in everyone and so if a virus emerges with a slightly different epitope, it will evade antibody in anyone infected with that virus. T cell epitopes are different. T cell epitopes are presented to T cells on the infected cell surface by MHC molecules, which are encoded by highly polymorphic genes. That means that your MHC is likely different from mine, and so will be the viral peptides displayed in them. So if a T cell epitope varies during your infection, it won’t matter to other people – their infected cells will be displaying different T cell peptides.
It is possible that SARS-CoV-2 will continue to produce altered spike proteins that will completely evade antibody neutralization. In this case T cells might not be enough to prevent severe disease – they could be overwhelmed by so many infected cells. Our rush to make vaccines – understandable given the urgency – have led us to such a situation. Most of the vaccines were based only on the spike protein. If we change the spike protein to accommodate variants, we might get in a never-ending cycle of changing COVID-19 vaccines on a regular basis. A better approach would be to produce second-generation COVID vaccines that include other viral proteins besides spike protein. Inactivated and attenuated vaccines fall into this category; another solution would be to modify authorized mRNA vaccines to encode additional viral proteins.

Hi Vincent, what a great article. From having listened to many of your TWIV’s over the year your group often seemed to talk about T-Cell responses and I wondered why I never heard about them from other sources.
On a personal note I am in the Pfizer study and had confirmation I was vaccinated in July and August last year. However had assumed covid (mild case) in November.
Keep up the good work you are doing! Thank you, Sincerely Scott
The link to the paper does not appear to work from my pc – I assume this is the Tarke et al La Jolle preprint? Thks
No kidding. That is why the DNA vaccine that I designed last year had all 4 of the capsule proteins. This solves that problem as you describe above. And being a DNA vaccine, it continues to produce small amounts of antigen in cells that escape killing by T-cells.
This didn’t get funding, in significant part due to the insistence by most working on the vaccine that ONLY the spike protein should be presented, and that if possible only the specific part of it that attached to ACE2 should be presented to make it “most effective”. The money people haven’t got a clue, and most vaccinologists are equally ignorant. If you surveyed physicians it would be rare that any could tell you what clears a virus, and it’s not antibodies.
It is also seen as problematic for testing to have a vaccine that present more than one protein. What the primary selected Ab’s are for a virus is stochastic, so not everyone will seroconvert for spike. Needing all 4 capsule proteins, or even just 2 is inconvenient for test makers because it requires culturing recombinant his-tagged proteins for everything, or else culturing the virus itself and using a fragmented version of that on the strips.
To say nothing about the intransigence of FDA and the field to the concept of T-cell testing. Ab’s as the only acceptable surrogate endpoint is a legacy of vaccines being the oldest form of modern medicine.
I believe that the Russian and Chinese vaccines are the attenuated and killed whole virus vaccines that you are describing. But even these will not keep up with the emergence of new variants in the Spike and other SARS proteins. The current mRNA vaccine approach can be used for rapid adaptation of the vaccine to emergent Spike mutants, so it will prove useful going forward. Meanwhile, I am disappointed that my CsCl recommendation, which targets the viral RNA polymerase and is thus insensitive to emerging mutations in the Spike and other proteins, and is furthermore active against negative-strand RNA viruses such as measles and flu, continues to be totally ignored.
Responding to Rch Bradley: I was able to unmangle the typo in the link, and yes it is Tarke et al: https://www.biorxiv.org/content/10.1101/2021.02.27.433180v1.full
They need a drug to block the translation of ORF1A.
Via the Ribosomes.
That would keep RdRp from being incorporated in the replicase scheme.
But if the vaccine prevents us from ending up in hospital and lets us leave with a light Covid, would it also prevent us from the long-term effects of Covid, like the likelihood of strokes and pulmonary embolism?
Thank you for all the things that I learned from your courses! Stay safe!
Sincerely,
Dora Szekeres
Pingback: COVID ‘Super Strains’ Got You Worried? Don’t Be, Says Top Virologist – Wary Fool
Pingback: COVID Super Strains? There Are None, Says Virologist Vincent Racaniello - Topzvirals
Pingback: COVID Super Strains? There Are None, Says Virologist Vincent Racaniello – Africa Investor Daily
Pingback: COVID Super Strains? There Are None, Says Virologist Vincent Racaniello - Financeusamagazine
Pingback: COVID Super Strains? There Are None, Says Virologist Vincent Racaniello - Bytdex
Pingback: COVID Super Strains? There Are None, Says Virologist Vincent Racaniello – Financial Advisers
Thanks Vincent for the great post. Isn’t this exact space, post antibody immune response (AKA T-cells to the rescue), where we need to be careful about ADE?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3335060/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7454712/pdf/jiaa518.pdf
The correct link to the bioRxiv paper is: https://www.biorxiv.org/content/10.1101/2021.02.27.433180v1
“The recent finding that amino acid changes in the spike protein of SARS-CoV-2 variants of concern do not impact T cell reactivity is very good news.”
Corrected link: https://www.biorxiv.org/content/10.1101/2021.02.27.433180v1
Pingback: Weekly COVID-19 UPDATE!!! – The Darkest Timeline
Unfortunately, this argument appears to be little more than the hubris of a lone, potentially rogue, scientist who feels slighted by the immunology community at large. For example, Dan, et. al. demonstrated in their Science article, “Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection,” both CD4 and CD8 levels begin to decline shortly after after infection, along with antibody levels, whereas B cell levels actually increase over time.