Vaccine development has far outpaced antiviral discovery for COVID-19. Hydroxychloroquine was a disaster, and the repurposed remdesivir, which must be administered intravenously, has modest effect when given to hospitalized patients. The situation is unfortunate because antiviral drugs may be used to either prevent infection (prophylactic) or treat infection (therapeutic). A promising antiviral drug candidate is EIDD-2801/MK-4482 which has been shown to block SARS-CoV-2 transmission among ferrets, and more recently, to treat or prevent infection in mice. In both cases the drug is active after oral administration.
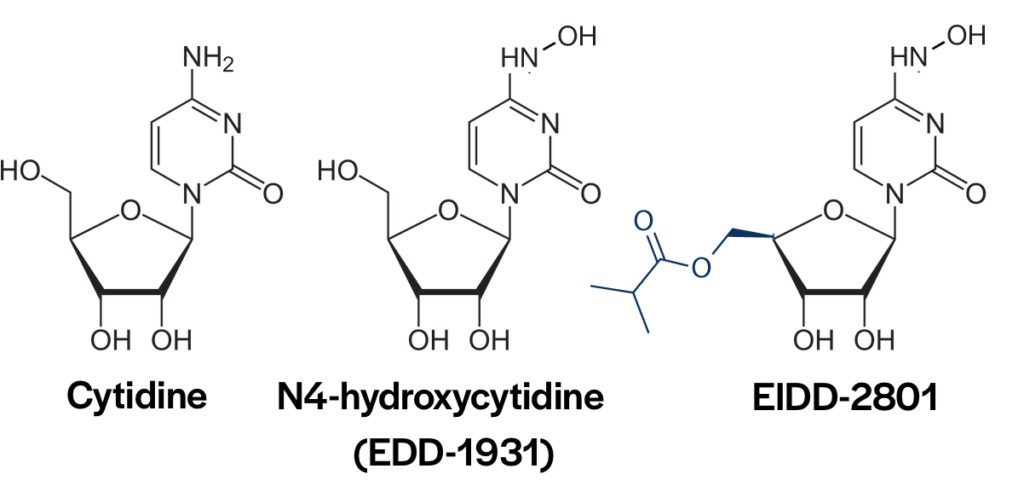

EIDD-2801 is a pro-drug of the nucleoside analog N4-hydroxycytidine (NHC) (pictured above). An earlier version of the drug, EIDD-1931, was not effective because of phosphorylation of the 5′-OH in intestinal epithelial cells. Addition of an ester group prevents phosphorylation. After oral uptake, EIDD-2801 enters the blood where the blocking ester is removed. NHC enters cells and is incorporated into RNA chains by the viral RNA-dependent-RNA polymerase. As a consequence, mutations are introduced in the viral genome leading to error catastrophe and loss of viral infectivity. Some years ago NHC was found to be a broadly-acting inhibitor of multiple RNA viruses, including coronaviruses and influenza viruses.
Activity of EIDD-2801 against SARS-CoV-2 has been recently examined in human lung-only mice (LoM). These mice are made by implanting human lung tissue into the back of immune deficient mice. The implants multiply and develop into a lung-like tissue that includes multiple respiratory cell types, alveolar sac structures, and blood vessels. When SARS-CoV-2 is inoculated directly into these lung implants, the virus reproduces primarily in ciliated epithelial cells and alveolar type II pneumocytes. Infection leads to cell death and infectious virus particles are produced.
To determine if EIDD-2801 can prevent infection with SARS-CoV-2, LoM mice were orally administered the drug 24 and 48 hours after infection and every 12 hours thereafter. Virus titers were reduced by 25,000 fold and 96%, respectively. When administered 12 hours before virus infection, and every 12 hours thereafter, virus titers were reduced over 100,000 fold. These observations support therapeutic and prophylactic efficacy of EIDD-2801 when given orally.
Phase 2/3 human trials of EIDD-2801 for treatment of COVID-19 are currently in progress. If shown to be effective, the drug will fill a substantial void in our antiviral armamentarium. It has been known since 2015 that EIDD-2801 inhibits the reproduction of other coronaviruses. One wonders how the trajectory of the COVID-19 pandemic might have been altered had EIDD-2801 been ready for efficacy trials against SARS-CoV-2 in early 2020.

Another possibility.
Here is a paper about Camostat, a cheap 35 year old generic drug — a pill — in clinical trials since April, 2020 as a treatment for Covid 19.  The drug inhibits a human protease enzyme, TMPRSs2, which the virus depends upon to infect human lung cells.  In the lab it can halt the infection. It also inhibits TNF-alpha, which has been implicated in the cytokine storm phase of the illness.
Since it acts against a human enzyme, rather than directly against the virus, it seems unlikely to be quickly defeated by viral mutations. Â
https://www.researchgate.net/profile/Mads_Kjolby/publication/346890219_Camostat_mesylate_against_SARS-CoV-2_and_COVID-19-Rationale_dosing_and_safety/
Clinical trials are in progress in Denmark, the Netherlands, Germany, the UK, the US, India, Japan, Mexico, Israel, Ireland and South Korea. Some of these trials should be about ready to print out. Hope it has succeeded.
Camostat is not a vaccine and it will never be a stock market darling because it is cheap and because any generic drug company is free to manufacture it. It is estimated that in India a course of treatment for one patient could be manufactured at a cost to the maker.of less than $1.50.
As noted It is a protease inhibitor. Bear in mind that In 1995 HIV was brought under control by a protease inhibitor.
Camostat needs to be used in combination with low dose hydroxychloroquine plus zinc.
Ivermectin meta-analysis shows it is helpful across the board, prevention, early replication stage and in the late pulmonary stage due to its anti-inflammatory properties. See Dr. Andrew Hill of WHO and Dr. Pierre Kory and Dr. Paul Marik of CCCAliance.com
Hydroxychloroquine IS effective when used prophylactically; the wide use of this and other quinine-based antimalarials may be the reason for the relatively low rate of COVID-19 in Africa. I have been advocating a trial of CsCl as a drug against RNA viruses. I have tissue culture data that it is effective against RSV and animal data that it rescues mice from lethal Sendai virus infection. The recipe is simple: Take a scant tsp of CsCl mixed in a glass of juice, wait ~8hrs, then eat a banana. Expect diarrhea. Repeat 3-4X.” CsCl is FDA approved for clinical use against certain aggressive cancers, so it should be safe to use against viruses. CsCl “bends” the N protein of the viral RNA polymerase, stalling viral transcription/replication thus allowing the host immune system to catch up and clear the infection. I am confident that CsCl will work against measles and flu, and speculate that it will also work against SARS-CoV-2 due to shared features of the viral nucleocapsid structures.