by Gertrud U. Rey
As of today, SARS-CoV-2 has infected 18.7 million people and caused 700,000 deaths worldwide. The most realistic way to quickly curb the spread of the virus would require daily identification and isolation of individuals who are contagious, a process that is hampered by cumbersome sampling and testing methods with slow turnaround times.
The predominant test for diagnosing SARS-CoV-2 infection is a highly sensitive assay called quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR). To carry out a SARS-CoV-2 qRT-PCR test, a mucus sample is processed to inactivate virus particles and extract the viral RNA. The RNA is converted to DNA (the reverse transcription step), which is then amplified during the polymerase chain reaction portion of the assay. For amplification to occur, a small piece of DNA (a primer) binds to a complementary target sequence in the SARS-CoV-2 DNA, while another piece of DNA (a probe) attaches to a sequence downstream of the primer binding site. Binding of the primer initiates amplification of the target DNA by an enzyme called polymerase, which copies the DNA in one direction towards the probe. Once the polymerase reaches the probe, it cleaves it, which activates a fluorescent marker attached to the probe. The use of this fluorescent probe allows for monitoring of the fluorescent signal quantitatively in real time rather than just detecting an accumulated end product.
While the qRT-PCR test is very sensitive, it also has multiple limitations. It requires expensive laboratory instrumentation and trained technicians with an estimated cost of about $100 per test, meaning that most people probably only get tested once. Current testing capacities are limited and results often take days or weeks to return, meaning that individuals who don’t know they are infected can transmit the virus during this time. The high sensitivity of qRT-PCR may also be a drawback rather than an advantage, because the test often detects small fragments of RNA that don’t originate from whole virus particles and thus don’t represent transmissible virus. Such RNA fragments can persist in individuals for weeks and months. As illustrated in Figure 1, infection with SARS-CoV-2 usually results in high initial levels of viral replication that peak and begin to decline within a few days. Symptoms don’t usually appear until after that peak has already occurred, and, because most people don’t get tested until they experience symptoms, they are likely already on the downward slope of viral replication and no longer infectious at the time of testing. In the meantime, they have been unknowingly transmitting the virus to others for several days. Clearly, these people need to be identified and isolated during their period of high infectivity.
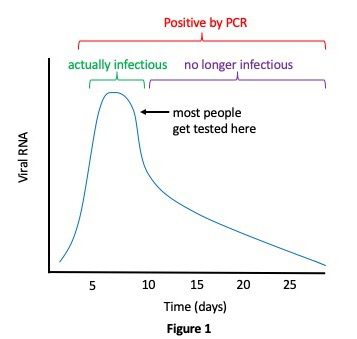

In late June, Harvard epidemiologist Michael Mina published a preprint that evaluates the effectiveness of current SARS-CoV-2 surveillance measures for reducing transmission when considering frequency of testing and delayed reporting of results. Mina and co-authors concluded that infrequent testing with an ultra-sensitive test like qRT-PCR often results in unnecessary quarantine of individuals who are no longer infectious. Notably, it also results in missing pre- or asymptomatic individuals who are at the beginning of their infection and thus highly contagious, allowing them to go about their daily routines and infect others.
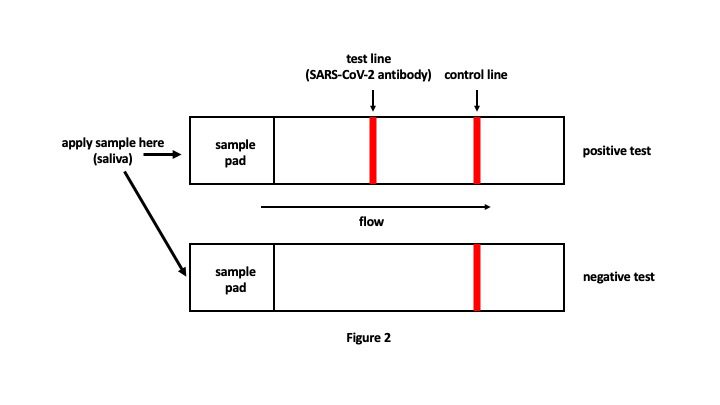
A few days after publication of the preprint, Mina co-authored an opinion article in the New York Times in which he discussed the potential for controlling the SARS-CoV-2 pandemic by widespread use of frequent, rapid at-home diagnostic tests. One example of such a diagnostic test is a lateral flow device, which is a paper strip that works similarly to a pregnancy test. The strip has a sample pad on one end and contains antibodies that recognize SARS-CoV-2 antigens. One would dip the sample pad portion of the strip into a sample of saliva and allow the saliva to wick across the strip. The presence of SARS-CoV-2 antigens in the saliva would be indicated by the appearance of a test line in addition to the control line, while a negative test would only indicate the control line (Figure 2). The test provides results in 10-15 minutes at a cost of about $1-2 per test and does not require any additional equipment. A positive result would indicate the need for self-quarantine and confirmation of test results through a doctor’s office.

Although these rapid tests are only about half as sensitive as qRT-PCR tests, they detect the presence of viral antigen during the actual window of transmissibility when viral levels are very high. The highly sensitive qRT-PCR assays detect viral RNA for weeks after a patient is no longer transmitting virus, which is irrelevant for quarantine/isolation purposes and does nothing to curb transmission. A less sensitive test that is done on a daily basis and provides immediate results would be more valuable because it would identify individuals while they are actually infectious. This would also alleviate the need for costly contact tracing measures because most infected individuals would be aware of their status and would stay isolated during their period of transmission.
Rapid lateral flow SARS-CoV-2 diagnostic tests are already available, but there is concern that the FDA may not approve these products because of their low sensitivity. You can help bring these products to market by writing to your elected officials (see sample letter templates here), contacting your local TV and radio stations, and telling your friends and family to do the same. Hopefully, with sufficient media attention, the FDA, CDC, and NIH will recognize the value of these tests and make them widely available to the public. This may be the ultimate solution for opening schools and workplaces, and for rebuilding the economy.
[This blog post is also covered in this video. Michael Mina discussed rapid at-home SARS-CoV-2 testing options on TWiV 640. Tidbits of that episode were also reviewed on MedCram.]

sounds a very good idea to me , what can i do topromote this in UK?
An excellent idea. It would be helpful to mention which companies make or quickly can make, the paper strip test you mention.
Thanks!
Here is the NYT article by Dr. Mina. It has contacts to the companies working on the quick, cheap tests. It would be great if some other more sensible govt. than the US would take up using the quick tests.
https://www.nytimes.com/2020/07/03/opinion/coronavirus-tests.html
This only works if 1) The claimed test can be produced, and 2) people bother to use it.
I keep asking for evidence of a test meeting the cost (including validation, production and distribution) and accuracy criteria necessary to have the claimed impact.
I get no response. How do we know someone isn’t trying to create another Theranos?
Theranos similarly claimed a test that could revolutionize the industry. It then tried to use their connections to do end-runs around regulators and control the media dialogue.
@Jon No one is trying to do an end run around regulators. The goal is get regulators to set criteria that are more appropriate for the real goal: detecting infectiousness. The tests could then be tested and approved in the usual way.
What progress are other countries having
with the testing being suggested by Mina?
@Jon, this is exactly the opposite of what Theranos did. Theranos was claiming that they had the technology to produce highly sensitive results very quickly and with very little material. These paper based tests are low tech and have pretty low sensitivity (although with high frequency use, the low sensitivity would not be an issue). This kind of test is exactly like the kinds of pregnancy tests that you can buy at any drugstore.