by Gertrud U. Rey

Does a first infection with SARS-CoV-2 make a person immune to a second infection? This question is one of the prevailing issues in the current pandemic.
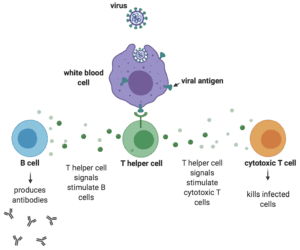
The relevant immune response is adaptive immunity, which initiates during a first exposure to a pathogen and protects from re-infection and disease upon a second exposure to the same pathogen. During that first exposure, T helper cells sense the presence of one or more proteins (i.e., antigens) on the surface of the invading pathogen and release a variety of signals that ultimately stimulate B cells to secrete antibodies to those antigens. However, antibodies only constitute half of the adaptive immune response. The other half – cell-mediated immunity – is just as important and at the very least results in activation of white blood cells that destroy ingested microbes and cytotoxic T cells that directly kill infected target cells.
The authors of a review published in the Journal of General Virology summarize some of the recent data obtained in regard to antibody responses to SARS-CoV-2 infection. They then compare these data to what is known about antibody responses to the six other coronaviruses that cause infection in humans. These viruses include the four endemic seasonal human coronaviruses – NL63, 229E, HKU1, and OC43 – as well as Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV, the virus responsible for the 2002-2004 SARS epidemic.
Most children produce antibodies to the four seasonal coronaviruses by the age of six. However, this antibody immunity likely wanes over time, because these viruses cause 22-25% of acute respiratory illness in adults. Even so, infection in adults results in low virus titers and usually only causes mild illness, suggesting that most infected individuals maintain at least some immune memory from their childhood. A study with human volunteers showed that ten out of fifteen adults inoculated with 229E virus became infected, and eight of these individuals developed clinical symptoms. All ten infected volunteers mounted antibodies that were neutralizing, meaning that they didn’t just bind viral antigens, but inactivated the virus and prevented infection of new cells. These neutralizing antibodies peaked at three weeks post-infection and dropped steadily until they reached baseline levels one year later. When previously infected subjects were intentionally re-infected with the same coronavirus at the one year mark, 66% of them became infected, but none developed clinical symptoms, suggesting that there was sufficient immune memory to prevent disease. Similarly, research with MERS-CoV and SARS-CoV shows that a primary infection with these viruses results in total binding antibodies and neutralizing antibodies, and that both types of antibodies decrease to a minimal detectable level by two to three years after infection.
Most of the SARS-CoV-2 antibody data obtained so far align with those observed with the other known coronaviruses, with most infected individuals having detectable antibodies by 10-14 days after onset of symptoms. One group studying antibody responses in hospitalized people in China measured these responses using three different assays. The first assay detected total antibodies to the receptor binding domain of the SARS-CoV-2 spike protein, the second assay measured IgM to the same antigen, and the third assay measured IgG against the SARS-CoV-2 nucleoprotein. IgM antibodies appear in the early stages of antibody-mediated immunity and typically bind very strongly to antigens, to the extent that they often cross-react with other, non-specific antigens. IgG antibodies arise later, are a lot more specific than IgM, and provide the majority of antibody-based immunity against invading pathogens. The results showed that 93% of patients produced total antibodies, 83% produced IgM, and 65% produced IgG, by about 11, 12, and 14 days after disease onset, respectively. More studies showing similar results are emerging.
The gold standard for determining whether a first infection renders a subject immune to a second infection is the “challenge” trial, in which a previously infected person is intentionally re-infected with the same pathogen. However, most experts consider human challenge trials for dangerous pathogens unethical, so these experiments are usually done in non-human primates. One such study in rhesus macaques showed that all animals infected with SARS-CoV-2 were protected from a second infection. Protection was mediated by both antibody and T cell responses and was demonstrated by mild clinical disease or no disease at all. Even though macaques are not people, their immune responses often parallel those of humans and can provide important insights into human immunity.
Although most human and animal studies suggest that exposure to SARS-CoV-2 elicits a strong immune response, some people don’t seem to produce antibodies after an infection. This anomaly may be due to the inaccuracy of existing antibody tests, which may lack sufficient specificity and sensitivity. In addition, there are many different tests available that are not calibrated against each other, a factor that may further contribute to inconsistencies. Currently available tests also don’t distinguish between total binding antibodies and neutralizing antibodies, a difference that can substantially impact evaluations of immunity and conclusions about whether immunity has been achieved.
Throughout the current pandemic, the public focus with regard to immunity has been on antibodies. This is partly because T cells are more difficult to measure than antibodies, and are thus unlikely to play a big role in evaluating immunity in previously exposed individuals. Nonetheless, because a well-balanced immune response requires both antibodies and T cells, there is presently no reason to believe that infection with SARS-CoV-2 does not provide at least some level of immunity.
The ideal immune response is “sterilizing” – meaning that it completely protects against a new infection. However, considering the current fatality rate of SARS-CoV-2 in some people, even a low level immune response that doesn’t protect against infection but prevents serious disease would be welcome. In other words, immunity is not binary and even partial immunity could potentially save a life.

Pingback: Immunity is not binary – Virology Hub
This is wonderfully written. Thank you.