A new drug has been approved by the US Food and Drug Administration for the treatment of influenza. Called Xofluza (baloxavir carboxyl), it is the first influenza drug approved in 20 years with a new mechanism of action.
How does Xofluza work?
Influenza virus particles contain (-) RNAs, which means that they cannot be translated into protein. When these viral RNAs enter the cell, they are copied by the viral RNA polymerase to produce mRNAs that can be translated.
The influenza viral RNA polymerase is a primer-dependent enzyme. The primers for influenza viral mRNA synthesis are produced from the cell€™s own collection of mRNA molecules. The influenza viral RNA polymerase consists of three different proteins.
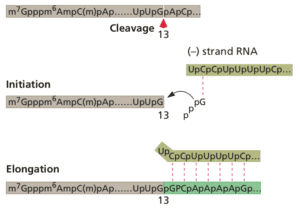

One of these, called an endonuclease, cleaves cell mRNAs near their 5€²-ends, generating the primers required for viral RNA synthesis. This process has been called cap snatching (illustrated), because the primers are made from the 5€²-ends of cell mRNAs, which have an unusual chemical structure called a ‘cap€™.
Xofluza (link to structure) is an inhibitor of the influenza viral endonuclease. In the presence of the drug, the endonuclease is inactive. Primers for the viral RNA polymerase cannot be produced, so no viral mRNAs are made, and infection is blocked.
Why was Xofluza approved by the FDA?
Xofluza underwent randomized, double-blind controlled phase 2 and 3 clinical trials to determine dosage and to test efficacy compared with an existing drug, oseltamivir. Patients with influenza-like illness were given a single oral dose of baloxavir, oseltamivir, or placebo for five days. Here are the key findings:
- Adverse events were reported in 20.7% of baloxavir recipients, 24.6% of placebo recipients, and 24.8% of oseltamivir recipients. These include diarrhea, bronchitis, nausea, headache, vomiting.
- Median time to alleviation of influenza symptoms in phase 2 trial was 23.4 to 28.2 hours shorter in baloxavir treated patients compared with placebo
- Median time to alleviation of influenza symptoms in phase 3 trial was 53.7 hours in baloxavir group compared with 80.2 hours with placebo
- Time to alleviation of symptoms was similar with baloxavir and oseltamivir
- Patients on baloxavir had greater reduction in viral load 1 day after dosing compared with oseltamivir and placebo
- Resistance to baloxavir was observed in 2.2% and 9.7% of phase 2 and 3 patients, respectively. Resistance was caused by amino acid changes in the viral endonuclease.
Baloxavir is a welcome addition to the influenza antiviral armamentarium. However, like oseltamivir, it must be given within 48 hours of onset of symptoms to be effective. It remains to be seen if resistance to baloxavir will be a problem when greater numbers of individuals are treated.
It would be of interest to conduct a clinical trial with both baloxavir and oseltamivir, which inhibit different steps in the influenza virus replication cycle (oseltamivir blocks the viral neuraminidase). Such two-drug therapy might solve the problem of viral resistance when using single drugs. Two-drug combinations have been used effectively to treat hepatitis C virus infections. The results of one study have shown that oseltamivir and baloxavir do not interact in a way that impacts their efficacy.

Pingback: A new drug for influenza – Virology
Pingback: A new drug for influenza - Vetmedics
I would be interested to know how this one day reduction in duration of illness compares to reduction in illness in people who have received the flu vaccine and still got sick. Which one works better, the vaccine or the therapeutic treatment?
Pingback: A new drug for influenza -
Trudy, if you think of the viral products as a pile of, say coal, that grows as time goes by at the exponential rate of viral propagation, then a one day truncation of the illness leads to a large reduction of body cells lysed by the virus – and these cells are mainly in the lungs, you end up with a large reduction in the amount of cellular damage and a large reduction in lysed cellular contents in the circulatory system.
Flu deaths are largely a result of this exponential growth phase (as are most viral deaths), during which the body tries to successfully mount an immune response and preserve the organism.