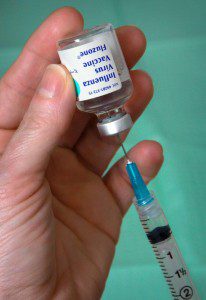

Because most influenza vaccines are grown in embryonated chicken eggs, and may contain residual egg protein, their use in individuals with egg allergy has been discouraged. However this practice is no longer necessary. The results of many studies have shown that inactivated influenza vaccine (IIV) is safe for people with egg allergies. Specifically:
- Presence of egg allergy is not a contraindication to receive IIV or LAIV.
- Influenza vaccine recipients with egg allergy are at no greater risk for a systemic allergic reaction than those without egg allergy.
- Precautions, such as choice of a particular vaccine, special observation periods, or administering in particular medical settings, are not warranted and constitute an unnecessary barrier to immunization.
- Vaccine providers and screening questionnaires do not need to ask about the egg allergy status of recipients of influenza vaccine.

Pingback: Influenza vaccine is safe for those with egg allergies – Virology