
Viral infections often lead to alterations in the energy-generating and precursor synthesizing pathways of a cell, to provide the building blocks and energy to produce new virus particles. The latest example is rhinovirus: infected cells mobilize glucose from extra- and intracellular pools, which is essential for viral replication.
Infection of cells in culture with rhinovirus causes augmented glucose uptake, an effect mediated by increased levels of plasma membrane glucose transporters. Analysis of infected cell metabolism revealed increased breakdown of glycogen to glucose; increased fatty acid synthesis (and decreased fatty acid oxidation); and increased production of nucleotide triphosphate. Rhinovirus infection therefore reprograms cellular carbohydrate, fatty acid, and nucleotide cell metabolism, presumably to permit the production of new infectious virus particles.
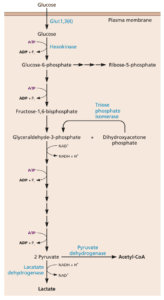
Glucose is central to these metabolic alterations. This sugar can enter the glycolytic pathway (illustrated), which leads to the production of energy in the form of ATP. Glucose can also enter the pentose phosphate pathway which gives rise to nucleotide biosynthesis. Finally, acetyl-CoA made from glucose can promote the synthesis of fatty acids.
That glucose is essential for rhinovirus replication was shown by depleting glucose from the cell culture medium, or by inhibiting glycolysis with 2-deoxyglucose. Both conditions caused reduction in rhinovirus replication. Treatment of infected cells with 2-deoxyglucose also prevented increases in the synthesis of fatty acids and nucleotides.
These findings demonstrate that rhinovirus infection induces a glucose-dependent switch towards anabolic metabolism, and inhibition of glycolysis prevents this shift.
Treatment of rhinovirus-infected mice with 2-deoxyglucose caused reduced lung inflammation and lower virus titers in this organ, compared with untreated mice. Animals treated with the glycolysis inhibitor displayed no visible side effects.
The authors conclude that metabolic starvation of rhinovirus with 2-deoxyglucose might be a viable antiviral approach. In general, interfering with host cell metabolic alterations induced by viruses might be a plausible target for antiviral therapy.

Comments are closed.