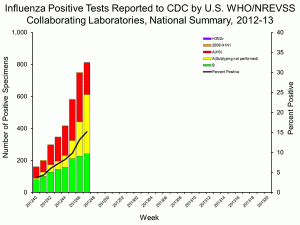

During week 47, 812 of 5,342 (15.2%) of respiratory samples tested positive for influenza virus. Of those isolates that were subtyped, most were either H3N2 or an influenza B virus strain. The 2009 swine-origin H1N1 strain has only been found in one sample so far. Fortunately the H3N2 component of the influenza vaccine for 2012-13 is a good match for the circulating H3N2 strain.
A substantial rise in the number of influenza cases typically does not occur until the end of December in the US. The last time that the disease incidence rose so early was in 2003-04. That was one of the most lethal seasons in 35 years, with 48,000 deaths. This year two children have already died of influenza in the US.
The good news is that 112 million doses of influenza vaccine have already been administered this year, and there is still time to be immunized. The CDC recommends that everyone over 6 months of age be immunized against influenza. Vaccination is especially important for individuals who are at risk for developing serious influenza-related complications: pregnant women, children younger than 5 years old (but those less than 6 months of age should not be immunized), people with with chronic medical conditions such as asthma, diabetes, and heart disease, and those over 65 years old.
The influenza vaccine is available in two types: an injected, inactivated preparation, and an infectious version that is sprayed in the nose. After immunization, approximately two weeks are required to be fully protected against infection.

You mean Dec 2-8 =)
Didn’t you know that I’m a Time Lord?