
The authors studied the effect of 45 compounds on the replication of XMRV in cell lines derived from human breast (MCF-7) and prostate (LNCaP) cancers. Twenty-eight of the drugs have been approved for use in humans, including treatment of HIV-1 infection. The drugs tested include nucleoside and non-nucleoside reverse-transcriptase inhibitors, and integrase and protease inhibitors. Other inhibitors used included aspirin, acylclovir (the anti-herpesvirus drug), and chloroquine (complete list here).
To test the ability of the drugs to inhibit XMRV replication, cells were infected with the virus, and increasing amounts of the compound were added to the cell culture medium. After incubation for six days, the virus in the culture medium was assayed by measuring reverse transcriptase activity. This enzyme, which converts RNA to DNA, is packaged within the viral particle. Its presence in the cell culture medium is therefore a measure of viral production. The cells were also monitored for cytotoxicity to ensure that a reduction in viral release was a consequence of specific antiretroviral activity, not toxicity of the compounds.
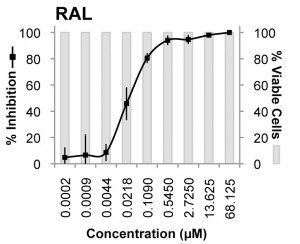
The most potent inhibitor of XMRV was raltegravir, the compound that blocks the viral integrase protein. This viral protein is essential for insertion of viral DNA into the host chromosome. The EC50 (the concentration of drug that inhibited virus production by 50%) was 0.005 µM in MCF-7 cells and 0.03 µM in LNCaP cells. Another integrase inhibitor, called L-000870812, also blocked XMRV replication, but at higher concentrations (EC50 of 0.16 µM in MCF-7 cells, and 0.7 µM in LNCaP cells).
Other drugs were found to inhibit XMRV replication at much higher concentrations. Among the most effective were zidovudine (ZDV; EC50 0.11 µM in MCF-7 cells and 0.14 µM in LNCaP cells) and tenofovir disoproxil fumarate (TDF). Protease inhibitors were less effective at blocking XMRV replication. Other non-HIV inhibitors were not effective at concentrations which were not cytotoxic.
The problem of drug resistance during treatment of AIDS was made manageable by using combinations of three antiviral compounds. Pairwise combinations of raltegravir, L-000870812, TDF and ZDV were tested for activity against XMRV in LNCaP cells.The effects were either additive or synergistic. All combinations that included raltegravir showed synergy without cytotoxicity. This observation is good news for treating XMRV infection. Based on the response of other retroviruses to combination therapy, the use of two antiviral drugs might suppress XMRV replication, reduce disease, and reduce emergence of resistant viral mutants.
How well do these drugs inhibit XMRV compared with HIV-1? The authors write that “Relative to HIV-1, the compounds were generally less potent against XMRV than HIV-1, especially at the EC90 level” – the concentration of drug needed to inhibit virus production by 90%. It’s difficult to predict from these data whether the drugs would be effective at effectively reducing XMRV levels in an infected individual. Chemical modification of the compounds could yield drugs that are more active against XMRV.
No matter what antiviral drugs are used, resistant viral variants inevitably emerge. For XMRV the process may be less problematic than for HIV-1. Compared with HIV-1, isolates of XMRV isolates have limited sequence diversity. The genomes of all the XMRV isolates obtained to date differ from each other at 27 out of 8,100 nucleotides. If this lack of diversity is a consequence of limited replication in the host, then the emergence of drug resistant variants could be significantly lower than HIV-1. A combination of two drugs might therefore be effective in treating XMRV infection. The authors note that after several months of propagating XMRV in the presence of raltegravir, drug resistant viruses have not emerged. But a human is very different from a dish of cultured cells.
Whether or not combinations of these drugs can be used to treat XMRV infection awaits the results of additional epidemiological studies, as well as clinical trials to determine efficacy. As the authors conclude:
If XMRV proves to be a causal factor in prostate cancer or CFS, these discoveries may allow for rational design of clinical trials.
Raltegravir has been previously shown to inhibit murine leukemia virus, which is highly related to XMRV. But the drug exacerbates autoimmune disease in mice which might rule out its use in treating CFS.
Ila R. Singh1, John E. Gorzynski, Daria Drobysheva, Leda Bassit, & Raymond F. Schinazi (2010). Raltegravir Is a Potent Inhibitor of XMRV, a Virus Implicated in Prostate Cancer and Chronic Fatigue Syndrome PLoS One : 10.1371/journal.pone.0009948

Thanks for the post. I'm really eager to know what role XMRV plays in prostate cancer and CFS since the studies came to so many different conclusions. We hopefully get the answer this year.
Your coverage on the topic of XMRV continues to be the best on the web – thank you!
Yes, thank you very much for the informative updates on Xmrv.
Why don't they just create a vaccine for this virus? It seems its sequence is very stable so they wouldn't have a make a new vaccine every year like the flu vaccine.
And I think it wouldn't take long to develop a vaccine because they were able to develop the swine flu vaccine quite quickly.
I'd love to hear podcasts or lectures about what's involved in creating a vaccine. Keep up the good work!
Pingback: uberVU - social comments
Thank you very much!
Thanks for another excellent post on this.
Pingback: Weekly PLoS ONE News and Blog Round-Up « everyONE – the PLoS ONE community blog
Hey Vincent – I'd love to hear more about why the sequence for this retrovirus is stable. Is the RT different or is it somehow biologically constrained/selected for? This is an intriguing observation…
Thanks again for another great article on XMRV. Again, as an XMRV+ CFS patient, your in depth discussions on this new retrovirus, particularly when it comes to treatment, gives me hope. I wish the clinical trials could start today!!!
Thanks again for another great article on XMRV. Again, as an XMRV+ CFS patient, your in depth discussions on this new retrovirus, particularly when it comes to treatment, gives me hope. I wish the clinical trials could start today!!!
Thank you for the excellent post. I would like to add however that the Beck-Ensenger paper reported raltegravir exacerbated autoimmunity in certain genetically susceptible mice that develop spontaneous autoimmune disease.
“We report here that the activity profile of raltegravir on the replication of murine leukemia virus is similar to that for HIV, and that the drug specifically affects autoimmune disease in mice, in which endogenous retroelements are suspected to play a role. While NZW and BALB/c mice, which do not succumb to autoimmune disease, are not affected by raltegravir, lupus-prone (NZBx- NZW) F1 mice die of glomerulonephritis more than a month earlier than untreated mice.”
Furthermore, it is quite possible that when combined with one or more effective reverse transcriptase inhibitors, this effect may be ameliorated. As the paper states:
“there apparently is a causal relationship between accumulation of retroelement DNA and autoimmune disease; the accumulating DNA triggers the type I IFN stimulatory DNA response.”
Jamie Deckoff-Jones MD
Hi. I own a medical practice in whitsett, NC. It is an Integrative clinic. I deal with dozens of chronic fatigue patients. A common theme seems to be chronic Epstein Barr as well as other viral pathogens such as mycoplasma, toxoplasmosis, cmv. I also find heavy metal toxicities in these patients using a provoked challenge test. I am very interested in the xmrv. I am going to order test kits and begin testing. Would be interested in hearing from patients who havehadsuccessful treatment with anti viral drug therapy such as valcyte. my clinic is Triad Wellness Center.