During a discussion about blogging on the Coast to Coast Bio Podcast, it was suggested that science professors should spend more time writing about their research – by explaining what problems they are trying to solve, how they approach them, and why they are interesting. My goal here at virology blog is mainly to teach virology. But explaining what we do in my virology laboratory can be an effective instructional tool.
About five years ago I became very interested in the innate immune response to viral infections. The innate response is considered the first line of immune defense because it is active even before infection begins. Many viral infections are halted by the innate immune system, which responds very quickly €“ within minutes to hours after infection.
The key to innate defenses are cellular proteins – toll-like receptors and cytoplasmic molecules – that can detect viruses and turn on pathways that lead to the synthesis of the antiviral interferons (IFN).
In 2005, I attended a virology meeting in Italy where I learned about two cellular proteins that can sense the presence of viral nucleic acids. I found it absolutely amazing that these cytoplasmic proteins – called MDA-5 and RIG-I – can detect viral RNA as a foreign molecule. When these proteins sense viral RNA, they interact with a mitochondrial protein called IPS-1, which then initiates a signal transduction cascade.
When I returned from that meeting I immediately began experiments to understand how our favorite viruses – poliovirus, rhinovirus, and encephalomyocarditis virus (EMCV) – interact with the innate defense system. To our surprise, we found that RIG-I, MDA-5, and IPS-1 are all degraded in cells infected with these viruses. The following figure shows sensing of viral RNAs (green) by these proteins, and the pathways that lead to IFN synthesis. The red arrowheads show which proteins are cleaved in virus-infected cells.
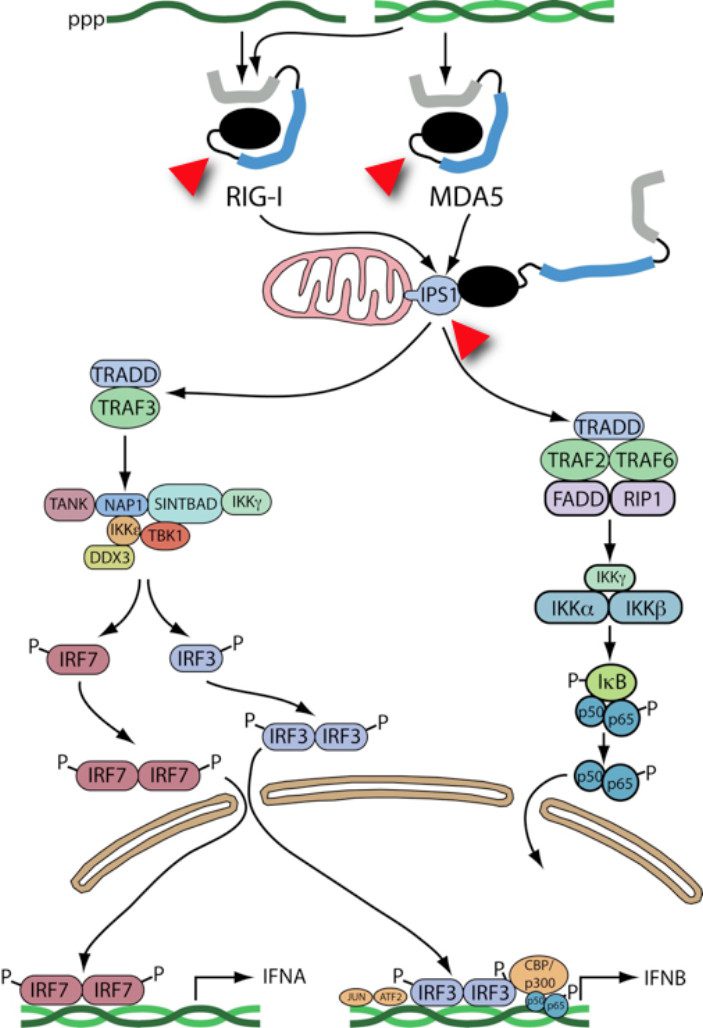

What does this mean? By eliminating these cell proteins, viruses could prevent the innate immune system from functioning properly – they could block synthesis of IFN. But we don’t have any evidence to support this hypothesis. RIG-I, MDA-5, and IPS-1 disappear quite late in infection, perhaps too late to impact IFN production.
A few days after our paper on the cleavage of RIG-I was published, another group reported similar findings. They found that in cells infected with EMCV, RIG-I is cleaved. In mouse cells lacking the RIG-I protein, normal amounts of IFN is produced after EMCV infection. They postulated that the presence of MDA-5 allowed viral infection to be sensed by the innate immune system. Next, they infected MDA-5-deficient cells with EMCV. In this case, no IFN was produced. This result made sense, they reasoned, because not only was MDA-5 absent, but RIG-I was cleaved by viral infection. To support this hypothesis, they infected cells lacking MDA-5 with EMCV, then added back new RIG-I protein to these cells. The addition of RIG-I lead to the synthesis of IFN, supporting their idea that both MDA-5 and RIG-I are important for sensing EMCV infection.
These results are still not conclusive – they do not prove that cleavage of MDA-5 and RIG-I antagonize the innate immune response. To prove this point, it is necessary to produce altered forms of the proteins that cannot be degraded in virus-infected cells. If cleavage of these proteins benefits viral replication, then synthesis of cleavage-resistant forms should lead to production of higher levels of IFN and reduced viral yields in infected cells.
I’m not sure if the results of these experiments would be worth the 6-9 months required for completion. We already know dozens of ways that viruses antagonize the innate immune system. I don’t believe that adding a few more mechanisms will substantially advance our knowledge of how viruses block immune responses. More importantly, I don’t think that these experiments are ‘fundable’. That is, it would be hard to convince the NIH to provide the money to do the experiments. There are other more interesting experiments that I’d like to do. But we’ll save a description of those for another time.
Drahos J, & Racaniello VR (2009). Cleavage of IPS-1 in cells infected with human rhinovirus. Journal of Virology PMID: 19740998
Barral, P., Sarkar, D., Fisher, P., & Racaniello, V. (2009). RIG-I is cleaved during picornavirus infection Virology, 391 (2), 171-176 DOI: 10.1016/j.virol.2009.06.045
Barral, P., Morrison, J., Drahos, J., Gupta, P., Sarkar, D., Fisher, P., & Racaniello, V. (2007). MDA-5 Is Cleaved in Poliovirus-Infected Cells Journal of Virology, 81 (8), 3677-3684 DOI: 10.1128/JVI.01360-06
Papon L, Oteiza A, Imaizumi T, Kato H, Brocchi E, Lawson TG, Akira S, & Mechti N (2009). The viral RNA recognition sensor RIG-I is degraded during encephalomyocarditis virus (EMCV) infection. Virology PMID: 19733381

this is also an area of interest to me too. however, that doesn't mean much since i'm only a grad student! 🙂
this is also an area of interest to me too. however, that doesn't mean much since i'm only a grad student! 🙂