There has been a great deal of discussion about the use of adjuvants to improve the immunogenicity of vaccines against the 2009 H1N1 pandemic influenza strain. What effect do these compounds have on the immune response?
Adjuvants are compounds added to vaccines that stimulate the immune response. They are often used when the antigen is in short supply, or does not induce a good antibody response. Because the 2009 H1N1 pandemic influenza strains do not replicate well in eggs, it has been suggested that adjuvants be used to ensure that there is sufficient supply of vaccine.
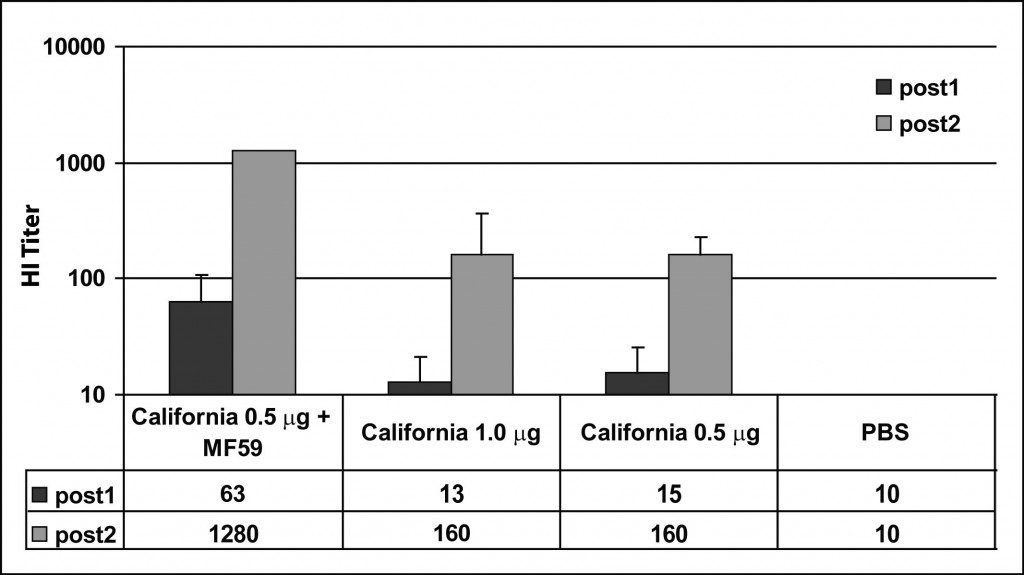
A recent study demonstrates very clearly the effect of adjuvants on the immune response. Mice were immunized with egg-produced 2009 H1N1 influenza vaccine with or without the adjuvant MF59. A boost inoculation was given on day 21. Sera were taken on days 13 and 21 and the antibody response was measured by hemagglutination-inhibition (HI) assay. If you don’t know how an HI assay works, please read my previous description. The results of the assay are shown in the figure.
One week after immunization with 0.5 micrograms of antigen, the average serum HI titer was 1:15 (bars labeled post1). This titer is barely higher than obtained when mice were immunized with buffer alone (PBS). The HI titer rose to 1:160 after the boost. When MF59 adjuvant was included, the first and second HI titers were significantly higher – 1:63 and 1:1280.
What do thes numbers mean? Protection of humans against seasonal influenza is generally believed to require a HI titer of 1:40 or more. Therefore when MF59 adjuvant is used in mice, one immunization is sufficient to confer protection against disease. Without adjuvant, two doses are required for protection.
Trials are ongoing in adults to determine the immunogenicity of 2009 H1N1 vaccines with and without adjuvant.
I know that many readers are concerned about the possible side effects of adjuvants. MF59 has been used for 12 years in seasonal influenza vaccines in Europe and is considered a safe adjuvant. However, the Centers for Disease Control and Prevention believes that the 2009 H1N1 vaccine will likely not be used with adjuvant.
Dormitzer, PR, Rappuoli, R, Casini, F, Wack, A et al (2009). Adjuvant is necessary for a robust immune response to a single dose of H1N1 pandemic flu vaccine in mice PLoS Currents: Influenza

While there's little doubt that MF59 would increase the response to 2009 H1N1 vaccination in people, there's a layer of complication here that makes this data less alarming than at first it seems.
Specifically, most adults aren't as naive about flu — in an immunological sense, of course — as the mice used in the Dormitzer report. And while the 2009 H1N1 doesn't appear to be recognized by antibodies specific for the pre-pandemic H1N1 strains, it's possible that CD4 help might cross-react, and this might support better antibody responses to a single-shot of 2009 H1N1 vaccine. And of course, wouldn't help the naive young.
Lots more needs to be — and is being — done.
Although Canada pre-paid GKS to supply the whole population with double doses, they're looking to run with the AS03 adjuvant.
Butler-Jones: “That means more vaccine for the rest of the world…Rather than hogging all of that material, it provides it for the rest of the world.”
Given the proven efficacy of various adjuvants in allowing dose reductions with specifically flu vaccines…the reluctance of various companies to adopt the strategy seems puzzling. Until you realise that use of ANY adjuvant requires a whole new set of clinical trials.
Seems to me that everyone should bite the bullet NOW, and determine which to use – because the old technology used for flu vaccine production is straining to provide enough for just the northern hemisphere, let alone for the next flu season in the south! We, of course, have mostly lived through the current winter flu season pandemic: you folk still have it coming….
Pingback: » Adjuvant effect on H1N1 vaccine H1N1SHOT.US
What is your opinion on below information?
Thanks in advance for your consideration.
Norvatis is the pharmaceutical company which is producing swine-flu vaccine for western countries. Squalene emulsified in Tween 80 is used in swine-flu vaccine as an adjuvant according to Norvatis patent, also used as immunization-sterility vaccine for domestic animals according to Ohio State patent.
Patent info:
SQUALENE EMULSIFIED IN TWEEN 80 SURFACTANT USED AS IMMUNIZATION –STERILITY VACCINE ON DOMESTIC ANIMALS:
http://www.wipo.int/pctdb/en/wo.jsp?WO=19840034…
WIPO Patent Application WO/1984/003443 Kind Code:A1
Modified polypetides capable of provoking the formation of antibodies in an animal may be produced by forming a linear polymer of polypeptide fragments, each having a molecular structure similar to a fragment of the protein to which antibodies are to be provoked. Such linear polymers can be made more immunogenic than the proteins to which they are related without introducing undesirable extraneous materials into the animal being treated, but have reproducable immunogenic properties. Proteins which are not endogenous or immunogenic to an animal can be chemically modified so as to make them more immunogenic. Also, modified antigens useful for fertility control can be produced by chemical modification of zona pellucida or sperm antigens. These modified polypeptides, antigens and modified antigens are desirably administered in the form of a vaccine having a vehicle comprising a mixture of mannide monooleate with Squalene and/or Squalene.
(Note: Tween 80 is a mannide oleate)
Original Provisional Patent:
FAYRER-HOSKEN, Richard, A. [US/US]; (US) (US Only). Inventor:FAYRER-HOSKEN, Richard, A.; (US). Agent:SANDBERG, Victoria, A.; Mueting, Raasch & Gebhardt P.O. Box 581415 Minneapolis, MN 55458-1415 (US). Priority Data:
60/070,375 02.01.1998 US
60/071,406 15.01.1998 US
60/076,368 27.02.1998 US
Title: FERTILITY IMPAIRING VACCINE AND METHOD OF USE Abstract: A vaccine comprising an antigen derived from a zona pellucida glycoprotein is effective to impair fertility in animals, preferably carnivores. The vaccine can be used as an immunosterilant or an immunocontraceptive.
FERTILITY IMPAIRING VACCINE AND METHOD OF USE This application claims the benefit of U. S. Provisional Application No. 60/070,375, filed January 2,1998, U. S. Provisional Application No. 60/071,406, filed January 15,1998, and U. S. Provisional Application No.
60/076,368, filed February 27,1998.
SQUALENE EMULSIFIED IN TWEEN 80 SURFACTANT USED AS ADJUVANT FOR SWINE-FLU VACCINE:
http://www.freepatentsonline.com/y2009/0208523…. (Norvatis ‘s patent)
Title:Adjuvant for vaccines
Document Type and Number:United States Patent Application 20090208523
Kind Code:A1
Abstract:
Vaccine containing a first vaccine, adjuvated with an oil-in-water emulsion comprising 5% squalene, 0.5% polysorbate 80 and 0.5% sorbitan trioleate in aqueous citrate buffer pH 6.5, and a nonadjuvated second vaccine as combination partners for the simultaneous, separate or phased application for immunization against viral, bacterial or parasitic infectious diseases.
Inventors:Broeker, Michael (Marburg, DE)
Application Number:12/386753
Publication Date:08/20/2009
Filing Date:04/22/2009
Attorney, Agent or Firm:
NOVARTIS VACCINES AND DIAGNOSTICS INC. (INTELLECTUAL PROPERTY- X100B, P.O. BOX 8097, Emeryville, CA, 94662-8097, US)
Claims:
1. A method for the contralateral administration of more than one vaccine composition, said method comprising: (1) administering a first vaccine composition comprising a selected antigen and MF59 to a subject; and (2) administering a second vaccine composition contralaterally to the subject, wherein said second vaccine composition comprises a selected antigen and does not include MF59.
(Note: MF-59 consists of 5% squalene, 0.5% polysorbate Tween 80, as described in the patent abstract.)
MF59 has been used in European influenza vaccines for years with no
side effects. It was tested in the new H1N1 vaccine in the UK in a
study published in the New England Journal of Medicine, 10 September
2009 ((10.1056/NEJMoa0907650).
So does this mean that without adjuvants the H1N1 vaccine will be essentially worthless since there will be no booster?
“….likely not be used…” tells me nothing that is relevant. Will, or will not the vaccine contain MF95?
I reacted badly to the MF59 (squalene) in the US Army's Anthrax vaccination back in 1990 and 1991. I will only take a H1N1 vaccination that contains no squelene.
If the VACCINE is ADJUVANTED, it could very likely contain (MF95/MF59 – Novartis) or (ASO3/ASO4 – GlaxoSmithKline), etc, etc. It depends on who gets the deal!
Which LEGAL DRUG MAKER gets the contract to supply the ADDITIVE (adjuvant).
Medical experts and scientists are working to develop a vaccine; it is expected to become available in the coming 2 months. In the meantime, effective treatments include regular influenza treatments and in severe cases anti viral drugs.
Novartis says pilot study suggest single dose of vaccine offers protection.
Vaccine producer release a statement on the result of the first (pilot) clinical trails, showing 80% by single dose of MF95 adjuvant pandemic influenza vaccine.
http://www.un.org.jo/index.php?page_type=specia…
Swiss company Novartis (NYSE: NVS), which is building a vaccine manufacturing facility in Holly Springs, received an order for $346 million for antigen and $343.8 million for adjuvant. London-based GlaxoSmithKline (NYSE: GSK), which has its U.S. headquarters in Research Triangle Park, received an order for $71.4 million of adjuvant.
http://triangle.bizjournals.com/triangle/storie…
Attorney Jim Turner, is filing a lawsuit in Washington D.C. against the Food and Drug Administration (FDA) in an effort to halt the distribution of the swine flu vaccine in America. The lawsuit charges that the FDA violated the law in its hasty approval of four swine flu vaccines by failing to scientifically determine neither the safety nor efficacy of the vaccines.
“The suit will seek an injunction against the FDA from approving the vaccine,†attorney Jim Turner told Natural News on Thursday evening’s Natural News Talk Hour show. “And the core of the argument is that they have not done the proper safety and efficacy tests on the vaccine to allow it to be release at this time.â€
Vaccine/Adjuvant combination has never been properly tested:
The vaccine/adjuvant combination, being referred to as the “swine flu vaccine†has apparently never been safety tested or approved by the FDA. In fact, in many cases the vaccine is being sent to clinics, pharmacies and other health establishments separately, from the adjuvant chemical, leaving it up to each local vaccine retailer to properly mix the vaccine with the adjuvant, according to information provided by Turner. With hundreds of millions of Americans potentially being targeted with this vaccine, the potential for improper mixing, improper dosages, and human error is alarming.
Normally, when a pharmaceutical achieves “FDA approved†status, there is a considerable paper trail of scientific scrutiny, peer review, clinical trials and other supporting evidence. To our knowledge, no such documents exist for the swine flu vaccines. The FDA’s approval of these vaccines appears to be based entirely on a whim.
“What has been tested?†asked attorney Jim turner. “Where has it been tested? Who reviewed the test? Who looked at the test and said yes they proved safety and efficacy? There is no record that we can find that shows these things have been done.â€
By approving the four vaccines in the absence of such safety testing, the FDA itself stands in direct violation of federal law. “There is a law that they’re supposed to follow and they are not following it,†Turner added.
http://www.naturalnews.com/027205_vaccines_swin…
HHS Purchases Additional H1N1 Vaccine Ingredients.
FOR IMMEDIATE RELEASE – July 13, 2009
Kathleen Sebelius – Secretary of the U.S. department of HEALTH & HUMAN services (HHS) announced; the department will commit $884 million to purchase additional supplies of two key ingredients for H1N1 vaccines. The funds will be used to place additional orders for bulk H1N1 antigen and adjuvant. The vaccine ingredients will become part of the pandemic stockpile, for use in the vaccination campaign.
http://www.hhs.gov/news/press/2009pres/07/20090713b.html
Not at all. The H1N1 monovalent vaccine is protective after one dose.
At the moment the H1N1 vaccine does not contain adjuvant. The first
doses reaching the streets are Flumist which is never mixed with
adjuvant. If you are concerned, take that vaccine. I don't know what
the US is planning but I believe that adjuvant will not be used in the
future as long as supplies of the vaccine keep up with demand.
Well, its like I said earlier…it all depends on the shot you're given.
Of all the vaccines A(H1N1) vaccines approved for use in the U.S., and or North America, three of them contain SQUALEEN (oil&water) based ADJUVANTS, they are: GlaxoSmithKline (GSK) “Prepandrix.” An Egg based production split cell virus containing the GSK (AS03) adjuvant. GSK boasts that more than 40,000 adults/children have received AS03 adjuvanted vaccines during development of seasonal & pandemic vaccines. Next, in the line up is the Sanofi-Aventis vaccine – “Humenza.” Formulated to contain 3.8 mcg hemagglutinin (HA) of influenza A/California/07/2009 (H1N1) v–like virus and includes AF03-ADJUVANT. Sanofi Pasteur’s proprietary adjuvant is aimed at stimulating the immune system to increase its response. And, last but not least is the infamous MF59 ADJUVANT, which many Gulf War Veterans can possibly relate to. The Novartis vaccine Celtura®, which was tested with 100 healthy volunteers, aged between 18 and 50 contains the MF59® adjuvant, which was well tolerated, pain at the injection site the most frequent adverse event.
So profvrr, what do you suspect your government's (HSS) gonna do with the 415 million worth of ADJUVANT they purchased with FEDERAL RESERVE NOTES….make salad dressings?
My apologies for being sarcastic.
soooo what's the consensus amoung you who sound way more educated than I – is the aduvant boosted H1N1 vaccine relatively safe?? I'm in Canada where I believe they are using an aduvant supported vaccine and this is causing me a lot of stress….to get the shot or not….Thanks!
The adjuvant being used in Canada is GlaxoSmithKline's AS03. I've
found several clinical studies comparing safety of influenza vaccine
with and without this adjuvant, and the only side effect specific to
the vaccine appears to be increased pain at the injection site. These
studies have involved several thousand individuals; it's hard to
predict what will happen when millions receive the adjuvant, but based
on these results I would take the adjuvanted vaccine.
Canada is also producing over 1 million doses of non-adjuvanted H1N1 vaccine for use by pregnant women. However, they say that, if the non-adjuvanted vaccine isn't available, these women should take the adjuvanted vaccine.
The use of a adjuvant has delayed the release of the vaccine until November to allow for extra time to test it.
I am also from Canada and am trying to decide between adjuvant and non-adjuvant vaccine (or no vaccine at all) for my young kids (age 3 & 1). Did any of those clinical studies you cited involve young children? It is one thing to decide what to do for myself, but to have to wade through the information to decide what to do for my kids is very stressful.
The Canadian aduvanted H1N1 vaccine will be available on Monday. A colleague mentioned that his md disclosed that a single dose would not provide adequate protection since it is 1/5th as strong as the unadjuvanted American vaccine. Can anyone speak to this? I have a hard time believing that Health Canada would give deliberately dilute vaccinations for the purpose of reducing the intensity of the illness, without clearly stating that purpose. Am I wrong?
My second question is about whether or not there is any truth to the rumors that squalene, one of the adjuvants has been linked to either infertility, rheumatoid arthritis or Gulf War Syndrome. There was an intellectual property patent for a pet population induced fertility vaccine showing online, but my assumption was that squalene was added for the same reason it is added to any vaccine, to boost the immune system response. Am I wrong?
My last question is that I read in the Globe and Mail that flu vaccines are not required to undergo human trials each year. So, what type of testing do they undergo then?
The adjuvant has been tested in young children – see my post on the Canadian H1N1 vaccine.
The adjuvanted Canadian H1N1 vaccine contains 3.75 micrograms of HA, while the non-adjuvated US vaccine contains 15 micrograms. The point of the adjuvant is to allow less antigen to be used. The Canadian vaccine has been tested in adults and been shown to induce a protective antibody response in over 90% of recipients. So the answer is 3.75 micrograms of HA plus adjuvant will provide adequate protection. I'll answer the other two questions separately.
With respect to testing of seasonal flu vaccines: in the US the FDA does not require clinical trials of the new vaccine that is produced each year, because they are made using the same manufacturing process except that the virus is slightly different (change in HA and NA antigens). The vaccines are tested for antigen content, sterility, and safety in a variety of laboratory animals.
There is not truth to rumors that squalene causes infertility, rheumatoid arthritis, or Gulf War Syndrome. As you state the compound is used to boost the immune response.
[The Canadian vaccine has been tested in adults and been shown to induce a protective antibody response in over 90% of recipients.]
CBC News – Tuesday, October 20, 2009 10:42 PM
Health Canada is poised to approve the H1N1 vaccine as early as Wednesday as Canadian tests of the vaccine against the swine flu virus get underway.
Canadian vaccine trials started this week, and the results won't be available until next year.
In the meantime, Canada's health regulator is relying on worldwide experience with producing flu vaccines and small European studies to approve the use of the vaccine in Canada.
http://news.ca.msn.com/top-stories/cbc-article….
A clinical trial in Europe by GlaxoSmithKline showed; 98 per cent of adults receiving a single shot, containing 5.25 micrograms of vaccine and, the company's AS03 adjuvant, had a spike in antibody levels thought to signal protection.
This trial does not answer the question; will the dose GSK hopes to sell to Canada, work?
http://www.thestar.com/article/695946
Squalene in an adjuvant formulation known as MF59, the secret ingredient in certain lots of experimental anthrax vaccine, caused devastating autoimmune diseases and death in countless Gulf War vets, including Canadian, British and Australian troops, also injected with squalene laced vaccine. It continues to be used today.
This adjuvant contributed to the cascade of reactions called “Gulf War syndrome.â€
The symptoms they developed included; arthritis, fibromyalgia, chronic fatigue, chronic headaches, dizziness, neuropsychiatric problems, anti-thyroid effects, systemic lupus erythematosus, multiple sclerosis and ALS (amyotrophic lateral sclerosis) also known as Lou Gehrig's disease. http://www.whale.to/vaccine/west_edda.html
Just one more flu vaccine.
The same manufacturer will be making the same vaccine and it's just considered as a (strain) switch. Health Canada, the U.S. Food and Drug Administration and the European Medicines Agency (EMEA) intend to treat the pandemic vaccine as a supplement or amendment to existing flu vaccines licences.
Governments and their vaccine regulators realized that if pandemic vaccines were treated like a brand new vaccine – The pandemic would be over before the pandemic flu shots would be injected into anyone.
Swine influenza in the U.S. in early 1976 led to fears a pandemic was emerging. More than 40 million people in the U.S. were vaccinated. The pandemic never materialized, roughly 500 people developed Guillain-Barre Syndrome (GBS), and about 25 deaths were attributed to the vaccination program.
http://healthandfitness.sympatico.ca/News/Conte…
Information compiled and provided by Christopher-Peter: Maingot; without prejudice, malice aforethought, ill will, vexation, or frivolity.
The GSK trial results of H1N1 vaccine with AS03 adjuvant provide strong evidence that it will work in Canada. What do you think would be a better way to show that the vaccine induces protective immunity?
The Sanofi vaccine was tested in children but it did not contain
adjuvant. I'll see if I can find the test results of the Canadian
vaccine with adjuvant, and if so I'll add to this discussion thread.
Do we really need another VACCINE?
Swine Flu Cases Overestimated?
CBS News Exclusive – October 21, 2009: Study Of State Results Finds H1N1 Not As Prevalent As Feared
http://www.cbsnews.com/stories/2009/10/21/cbsne…
Obama declares swine flu a national emergency.
The Associated Press, cbc.ca, October 24, 2009
http://www.cbc.ca/health/story/2009/10/24/flu-o…
Is there a new pandemic in town?
By Dr. B. M. Hegde, India [August 20, 2009]
I wonder as to how much longer will medicine’s flagship educational events (like warning of epidemics) fly the colours of the drug industry?
A new rapid (rRT-PCR) test which, notably, is designed by the same pharmaceutical company that also has the world wide rights for selling Tamiflu.
http://mangalorean.com/browsearticles.php?artty…
Applied Biosystems Receives FDA 510k Clearance for Real-time PCR Instrument.
http://www.highbeam.com/doc/1G1-187191884.html
The multifaceted arrangement between Applied Biosystems and Roche.
http://www.in-pharmatechnologist.com/Industry-D…
Government virus expert paid £116k by swine flu vaccine manufacturers
27th July 2009
Read more: http://www.dailymail.co.uk/new…z0UD0w27O1
Information compiled and provided by Christopher-Peter: Maingot; without prejudice, malice aforethought, ill will, vexation, or frivolity.
No offense intended: I'm simply not in favour of vaccines at all. This includes the notion of whether they provide “protective immunity.” I do know this however; when the Japanese health department adjusted their vaccine schedules for infant children; whereby they delayed administering vaccines to children under six years of age, the incidence of Sudden Infant Death Syndrome (SIDS) dropped by more than 50%.
I'm more inclined on a cleaner environment in which people are required to live. And, I also believe that we can all benefit, immunologically, from healthier diets, consisting of foods that actually contain nutritional value. So, I'll say it here right now, I think the idea that Bill Microsoft Gates has, in regard to GM (genetically modified) Foods, for the African continent, was as good of an idea as was his VISTA. If the foods that we are expected to eat cannot support it's own seeds, then how will it be able to support us?
If you don't mind me asking ..what type of reaction did you have..I am a concerend mother from canada who is worried about the squalene.
I'm in Canada too..and share your worries…I went into scholarly journals and found a lot of ocntroversary on adjuvants within the scientific world (not the internet world of information) so I am really confused as to why they are the only option in canada.
The suggestion that vaccines do not provide protective immunity has been well established and is beyond dispute. Their use has helped to extend the average human lifespan from about 30 years old to over 80.
Thank you for answering all of my questions. Just thought I would add one additional comment. My family and I had the H1N1 adjuvanted vaccine yesterday, and I ended up spending the night in emergency (surrounded by H1N1 patients) being monitored for an adverse reaction classified as oculo respiratory syndrome. Go figure! Apparently this symptom is rare, but there have been clusters of people reporting it in our province (AB) and the neighboring province (BC). as well, I have to say, everyone whoI have spoken with who had the shot yesterday agrees that their arm is much sorer than the regular flu shot.
I thought I read on the WHO website that although people make the association or claims regarding the Gulf War Syndrome due to squalene, they actually didn't even use squalene in that particular vaccine.
I find your post confusing, as it seems to directly contradict the previous replies.
I would like to suggest; that you perhaps re-direct your question regarding – Squalene/GWS/Anthrax Vaccine, to “profvrr.”
Perhaps he can better explain which people [make the association or claims] and, whether there was [squalene in that particular vaccine].
The following is just additional information which you may find to be of interest.
Flu Shot Reactions Worry Officials.
Almost 1,000 Canadians have suffered adverse reactions to the flu vaccine in the past two months, Health Canada has revealed – [dubbed oculo-respiratory syndrome].
http://vran.org/vaccines/flu/flu-worry.htm
Analysis of Relative Risks and Levels of Risk in Canada.
Scroll down to Table 3. and take note of the first eight or so causes of death.
http://www.enerex.ca/articles/risk.htm
I honestly hope, that I've not now added to your confusion…
Pingback: Has an Israeli Company Come Up with a Cure for Swine Flu – and All Other Flus, Too? | israeltech blog
Thanks for the info Profvrr. I'm researching what to do for my 6month old and 2 year old children. Everything I'm looking at shows little confirmation in safety in the adjuvant's use in children. The canadian government recommends the adjuvant vaccine but there is an option to use the unadjuvant vaccine in children. I'm wondering if this the same as a non adjuvant vaccine used by the Americans and if it is as effective as the adjuvant vaccine.
Thanks for the info Profvrr. I'm researching what to do for my 6month old and 2 year old children. Everything I'm looking at shows little confirmation in safety in the adjuvant's use in children. The canadian government recommends the adjuvant vaccine but there is an option to use the unadjuvant vaccine in children. I'm wondering if this the same as a non adjuvant vaccine used by the Americans and if it is as effective as the adjuvant vaccine.
My arm was sore as well….BUT, I am much better off than the folks upstairs in ICU on VENTILATORS!
I had an injection for H1N1 this am and within 2 hours devoped swollen eyelid.
should i expect to have further symptoms – did you.
Keep an eye out for other symptoms….my understanding is they will dissappear in 24-48hours and are mild for the most part.
You said: “I do know this however; when the Japanese health department adjusted their vaccine schedules for infant children; whereby they delayed administering vaccines to children under six years of age, the incidence of Sudden Infant Death Syndrome (SIDS) dropped by more than 50%.”
Can you please provide the data supporting this claim?
See page 2 on this link:
http://eugenechiropractor.com/images/Crib_Death…
Some other info found during my search to find this material (data) for you:
http://www.vaclib.org/basic/japanusa.htm
(d) JAPAN In 1975, about 37 Crib Sudden Deaths were linked to vaccination in Japan. Doctors in one prefecture boycotted vaccinations, and refused to vaccinate. The Japanese government paid attention and stopped vaccinating children below the age of two years. When immunization was delayed until a child was 24 months of age, Sudden Infant Death cases and claims for vaccine related deaths disappeared. Japan zoomed from a high 17th place in infant mortality rate to the lowest infant mortality rate in the world when they stopped vaccinating.
http://www.consumerhealth.org/articles/display….
“Delay of DPT immunization until 2 years of age in Japan has resulted in a dramatic decline in adverse side effects. In the period of 1970-1974, when DPT vaccination was begun at 3 to 5 months of age, the Japanese national compensation system paid out claims for 57 permanent severe damage vaccine cases, and 37 deaths. During the ensuing six year period 1975-1980, when DPT injections were delayed to 24 months of age, severe reactions from the vaccine were reduced to a total of eight with three deaths. This represents an 85 to 90 percent reduction in severe cases of damage and death. (Ref21).”—Raymond Obomsawin, MD
http://www.whale.to/m/quotes17.html
I hope it may suffice, in support of “the claim.”
I have a few more questions. 8 hours after receiving the flu vaccine ended up in emergency late Monday night (early Tuesday morning) with the oculo respiratory syndrome. On either side of my emergency cubicle divided by curtains, were confirmed, very ill, H1N1 patients, coughing. I would guess that their proximity was less than five feet on either side.
What are the chances that my husband and I will contract H1N1?
So far we have not developed any flu symptoms (Friday night), but if we did, would our symptoms be less fatal because we have likely developed “partial” antibodies since Monday 5pm?
Am I also correct that we have to wait until the following Monday night to know whether or not that close exposure would result in virus transmission?
I mean, we didn't touch anything, but we weren't wearing masks, and neither were the health care workers who aided us.
Thanks
Not to be inflammatory. But I can't imagine any supporting data would be available to substantiate such a lofty claim. I find that when information is presented without any level of uncertainty present, there is an inverse relationship with its credibility.
Again, I don't mean to sound disrespectful, but it seems obvious that it takes a great deal of scientific planning and rigorous testing to substantiate a causal relationship because of the myriad and multitude of variable involved, alone.
My son and I got our flu shots and H1N1 shots on Tuesday, October 27, how do I know if it had the adjuvant and if it did not will we need another shot???
Afraid of the flu – or afraid of the shot?
http://www.conspirare.net
During the last few weeks, I have been trying to find an answer to this question.
I started reading about the ones that voice concerns.
Dr Kent Holtorf, an Infectious Disease Expert wouldn’t even consider having his family take
the vaccine. Glenn Beck from Fox News asks why the World Health Organization, Big Pharma,
and the government are hyping a pandemic and he sais that he would do the exact opposite of
what the government recommended and attend a swine flu party. Dr. Mayer Eisenstein is
advising his patients to say no and is convinced that vaccines cause autism and health care
workers are protesting the flu vaccine mandate. A a growing chorus of doctors and
researchers is claiming that being injected with the swine flu vaccine may be more hazardous
than catching the flu and two-thirds of parents have serious reservations and Billy Corgan,
poet, rocker and H1N1 sceptic, believes that 'this virus was created by man, mostly to scare
the hell out of people.'
I then took note of legal action against the vaccine.
Jim Turner, a Washington, D.C., attorney noted for his work on consumer health issues, filed
in federal court to stop all swine flu vaccinations' because none of the vaccines against
H1N1 have been properly tested, Austrian Journalist Jane Burgermeister filed charges against
WHO and UN for Bioterrorism and Intent to Commit Mass Murder and Rebecca Campbell filed a
federal lawsuit stating that there 'exists a bank-based transnational corporate criminal
conspiracy to violate the fundamental constitutional rights'.
And what about the official position on this issue?
A former federal health minister dismisses as crackpots and conspiracy theorists those who
would actively discourage Australians from having their swine flu vaccine and experts assert
that the Swine flu vaccines are safe and time-tested. The WHO Denies Reports of H1N1
Vaccine’s dangerous Effects and informs that the side effect of administering H1N1 vaccine
is limited to a slight fever and pain at the site of injection. France, Greece the UK and
the US have or are planning to make the Swine Flus vaccination mandatory. And the German
Government will get a special swine flu vaccine. An official UK government report is warning
the British public that there will be countless deaths and that freight containers and
inflatable storage units may be needed to provide extra mortuary space.
And what about the money the Big Pharma makes?
Novartis, GlaxoSmithKline, MedImmune, Australian drug maker CSL, and Sanofi-Pasteur will
likely make a great deal of money, there is no financial incentive to make a safe vaccine,
and no repercussions for making an unsafe one and Drug companies have already sold $1.5
billion worth of swine flu shots.
And finally I read about Desiree Jennings who can move sideways and backwards, but not
forward.
So, should I be afraid of the Swine Flu, of the vaccine – or of both?
Right now, I'm just surprised. Surprised that these vaccines contain so many additives,
surprised I see no freight containers and inflatable storage units to provide extra mortuary
space – yet. And surprised that if this vaccine is so safe and time-tested and the side
effect of administering H1N1 vaccine is limited to a slight fever and pain at the site of
injection Jim Turner, a Washington, D.C., attorney, Austrian Journalist Jane Burgermeister
and Rebecca Campbell from Seattle have deemed it necessary to file serious charges.
It just does not fit together. Something is wrong. Maybe something is wrong with this global
vaccine campaign. Maybe something is wrong with my perception of it.
But definitely it seems wrong to me to force me to get this flu shot. And it seems wrong to
me to force anybody to get this flu shot.
And I find it strange that the WHO, that the Big Pharma, that the governments seem to be
very unable at informing the global public in a way that those fears are dispersed.
Why should the Government get a different vaccine than my daughter? And why should my
daughter get a vaccine with so many potential side effects for a flu that has killed –
comparatively – so few people? (Currently around 5000 worldwide)
And what are the long term consequences of such a vaccine? Is this good for my immune
system, does this vaccine protect me from a mutated swine flu virus? Is this vaccine going
to increase my dependency from Big Pharma to survive? Is autism possibly really caused by
vaccines?
And how can a few weeks be enough time to test such a vaccine?. Make sure it is safe to use
for children? Safe for my wife? Safe for me?
I'm waiting. Still waiting for information that could comfort me. That could remove my fear.
Information that could answer more and more questions I have.
Will the governments, the WHO, the media, the Big Pharma be able to provide me this
information?
Might I and YOU not have a constitutional right to be properly informed? Instead of forced?
Too many questions. Too few answers. This seems to be the Swine Flu Game.
The above content contains many references to articles in the press. Of course, the authors
of these articles may be right, wrong or influenced.
Read the full notepad with media references @ http://www.conspirare.net
notepad publishing (a non-profit news agency based in Switzerland)
I might have to assume now, that you have taken to censoring any of my comments.
It appears obvious, that you are not prepared to post any new ones, which I submit. It can’t be that they are too long in content, given the last post by; “notepad publishingâ€.
This control, which you can obviously exercise over your forum, is surely your prerogative…it seems odd, that all of a sudden, you now have to approve every comment I submit. This was not really the case in my previous comments, posted, at least for 99% anyway. You did seem to censor some, and were not willing to post them, for some reason. This might only leave me with the assumptive impression, that you're being a bit one sided.
[would our symptoms be less fatal because we have likely developed “partial” antibodies]
loladitka: fatal usually refers to having died…less than that should be; still alive…can’t be a little bit dead.
Information compiled and provided by Christopher-Peter: Maingot; without prejudice, malice aforethought, ill will, vexation, or frivolity.