Centers for Disease Control and Prevention have determined that some adults have serum cross-reactive antibodies to the new influenza H1N1 virus. One of the techniques used to reach this conclusion is the hemagglutination inhibition (HI) assay. How does this assay work?
To understand the HI assay, we must discuss the hemagglutination assay. Influenza virus particles have an envelope protein called the hemagglutinin, or HA, which binds to sialic acid receptors on cells. The virus will also bind to erythrocytes (red blood cells), causing the formation of a lattice. This property is called hemagglutination, and is the basis of a rapid assay to determine levels of influenza virus present in a sample. To conduct the assay, two-fold serial dilutions of a virus are prepared, mixed with a specific amount of red blood cells, and added to the wells of a plastic tray. The red blood cells that are not bound by influenza virus sink to the bottom of a well and form a button. The red blood cells that are attached to virus particles form a lattice that coats the well. The assay can be performed within 30 minutes, and is therefore a quick indicator of the relative quantities of virus particles.
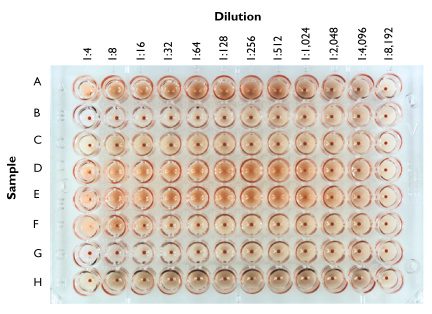

In the figure above, two-fold dilutions of samples of different influenza viruses (A – H) were prepared, mixed with chicken red blood cells, and added to the wells of a 96-well plate. After 30 minutes the wells were photographed. Sample A causes hemagglutination up to the 1:256 dilution; therefore the HA titer of this virus stock is 256. The sample in row B contains no detectable virus, while that in row D has an HA titer of 512.
The HA assay can be easily modified to determine the level of antibodies to influenza virus present in serum samples. In the CDC study cited below, the authors wished to determine whether stored serum samples contained antibodies to the new influenza H1N1 strain. First they obtained a preparation of one of the new influenza viruses, specifically A/California/04/2009 and determined its HA titer by the method described above. They added a fixed amount of virus to every well of a 96-well plate, equivalent to 32 – 64 HA units. Then they prepared two-fold dilutions of each serum to be tested, and added each dilution series along a row of wells. Finally, they added red blood cells and incubated for 30 minutes.
The basis of the HI assay is that antibodies to influenza virus will prevent attachment of the virus to red blood cells. Therefore hemagglutination is inhibited when antibodies are present. The highest dilution of serum that prevents hemagglutination is called the HI titer of the serum. If the serum contains no antibodies that react with the new H1N1 strain, then hemagglutination will be observed in all wells. Likewise, if antibodies to the virus are present, hemagglutination will not be observed until the antibodies are sufficiently diluted.
The CDC report contains the statement “…serum HI antibody titers of 40 are associated with at least a 50% reduction in risk for influenza infection or disease in populations”. A serum HI antibody titer of 40 means that at a dilution of 1:40, but not higher, the serum blocked hemagglutination. By determining HI titers and comparing them with influenza attack rates in populations, it is possible to calculate the significance of the HI antibody titer with respect to susceptibility to influenza virus infection. When used in this manner, the HI assay is a powerful epidemiological tool.
J Katz, PhD, K Hancock, PhD, V Veguilla, MPH, W Zhong, PhD, XH Lu, MD, H Sun, MD, E Butler, MPH, L Dong, MD, PhD, F Liu, MD, PhD, ZN Li, MD, PhD, J DeVos, MPH, P Gargiullo, PhD, N Cox, PhD (2009). Serum Cross-Reactive Antibody Response to a Novel Influenza A (H1N1) Virus After Vaccination with Seasonal Influenza Vaccine Morbid. Mortal. Weekly Rep., 58 (19), 521-524
Potter, CW, & Oxford, JS (1979). Determinants of immunity to influenza infection in man. Br Med Bull, 35, 69-75

Thank you for the information
Can you please explain the difference between one-hit kinetics verses two-hit kinetics. Its often mentioned in plaque assays. Thank you.
Presumably you saw my recent post on this question at virology blog.
In a plaque assay, one-hit kinetics means that one virus particle is
sufficient to form a plaque; for two-hit kinetics, two particles are
needed.
This was a wonderful and clear explanation; thank you!
I am wondering if there is a test available to check for H1N1 antibodies.
Yes, there are assays for antibodies to H1N1 virus. One is the
hemagglutination-inhibition assay which we discussed here:
https://virology.ws/2009/05/27/influenza-hem….
Thank you for answering so quickly! This assay is where I saw the test, but am wondering if there is one available to the public to rule out the absolute need for vaccination due to allergic reactions.
Some years ago an ELISA kit was developed to detect antibodies against
H1N1 swine influenza (not the current strain). You can read about it
here: http://www.jvdi.org/cgi/reprint/16/3/197. I believe the product
is HerdCheck Swine Influenza Virus H1N1 Antibody Test Kit, IDEXX
Laboratories, Inc., Westbrook, ME. I am not aware of a similar kit to
detect antibodies to the 2009 H1N1 strain.
Pingback: Pandemic H1N1 influenza virus outcompetes seasonal strains in ferrets
Very interesting and useful
Very interesting, clear and useful
Pingback: H1N1 Vaccine Study Summaries: Single Dose Provides Protection | Highlight HEALTH
Pingback: TWiV 50: XMRV
Pingback: SCIENCEPODCASTERS.ORG » This Week in Virology #50: XMRV
Do you know if membrane glycoproteins having the preferred receptor for influenza A and B virus ( with N-acetylneuraminic acid (NANA) as terminal units) are commercially available? I am working on the development of drugs blocking the receptor and need an assay indicating this mechanism of action.
Thanks,
Migmuz
We used to use fetuin, which is commercially available, as a
neuraminidase substrate.
Thanks a lot for the promptly response. Incidentally, your book (Principles of Virology) is exceptional. I borrowed a copy from a colleague to study about the attachment mechanism and end-up reading from all chapters. It’s a must have!
Migmuz
Thanks for an informative posting.
Does anyone know of a commercial or non-profit entity that can run hemagglutination inhibition assays to determine the level of influenza virus antibodies in human serum samples? We are an academic center that does not have this capability, and so we need to outsource. Many thanks.
There are many outsourcers for HI assays. Here is one:
http://www.southernresearch.com/contract-servic….
The assay you request and other studies are also offered by IIT Research Institute in Chicago; http://www.IITRI.org.
Pingback: This Week In Virology « Influenza A (H1N1) Blog
Pingback: This Week In Virology – Esta semana en virologÃa « Gripe por A (H1N1) Blog
I would like to get an antibody titer done for h1n1 but I do not see that doctors test for this before giving the h1n1 vaccine. I believe I have already had h1n1 and would ike to know if I truly need the vaccine.
As you probably have read in the news, physicians are swamped just
dealing with the number of people who would like to get the H1N1
vaccine. It's highly unlikely that you could convince them to test you
for antibodies to the virus. Physicians cannot do tests for antibody;
this requires a specialized laboratory. If you have already been
infected with the new H1N1, getting the vaccine will not cause a
problem. If you have not been infected, the vaccine will protect you.
Pingback: Influenza A (H1N1) 2009 vaccine: efficacy and safety
What exactly is the list of ingredients in the H1N1 vaccine?
(Does it contain any blood, blood products, red cells or white cells)
Pingback: Being older is a good defense against 2009 H1N1 influenza virus
I have a 9 year old son who's about to get the vaccine at his school. There hasn't been many cases of H1N1 virus in the area we live. My question is:
Should we wait until there's more of a threat to vaccinate our son?
He has allergies ( grass, trees, mold, dust mites, cats,etc…..).
I have to make my decision before 11/10/09. What are the side effects?
Do you think that if we use different kind of blood like turkey, horse, human blood
will it give a better results?
You should have your son tested for egg allergies before receiving the
vaccine. In those without allergies the side effects include injection
site soreness, fever, aches, chills. The vaccine should not be given
to those with egg allergies.
Thanks for the reply. My physician said they will test me but I have to find a lab. Any suggestions?
An allergist is a good option. Check
http://www.acaai.org/LocateAllergist/ to find one.
could you please suggest also an outsourcer for flu challenge studies in mice?
I am attempting the same thing you are- did you get it done and how?
Jeanie-
I can't seem to find a lab that will do the “titer” just the PCR for the H1N1 influenza virus (and not the antibodies).
I am still looking and will keep you posted if I find anything. I am checking with my allergist on Monday to see what he says. My internal medicine doctor is of no help in finding a lab to do this.
Sincerely and stay well
Diane
Sounds like the biggest threat is getting the H1N1 virus in the younger population (cdc recommends 2 vaccinations for children under 9 and antibody response takes time remember!) I would vaccinate if it were my child as soon as the vaccine is made available. Where do you live? I listened to a great radio program on NPR the other day with a panel of doctors discussing the virus/vaccine. I'll post a link when I find it.
Best Wishes
Diane
My 2 year old niece received her 1st H1N1 vaccine yesterday. She slept quite a bit more than usual the first 24 hours but other than that she is doing well. Long term effects unknown.
I believe there are many in the community who have contracted the novel H1N1 swine flu infection but not had confirmatory testing at the time of acute infection. They may, subsequent to recovery, be faced with the recommendation (particularly if in a high-risk demographic) to uptake the vaccine to protect self (if they actually had some other non-novel H1N1 illness) or general community or specific groups with which they have contact (at work, etc).
To take the vaccine if one has already acquired natural immunity offers no benefit that I can perceive to anyone (other perhaps than allaying anxiety of health/other administrators – but even this is spurious relief, and increasing use of a commercialized health care product). Not having the vaccine if truly not needed would limit wasteful use of a (still) scarce and important resource, direct such use to the more appropriately targeted, enhance to the public the credibility of health care authorities and fulfil more satisfyingly principles of parsimonious distribution of public and individual health care in a time of epidemic.
Finally although the likelihood and severity of adverse reactions to the novel H1N1 vaccine (in the non-allergic) is reported as low, the avoidance of such is surely a desirable goal in those who do not need the vaccine.
The above argues for the ready availability of post hoc serological testing but my question is whether a (presumably single) “convalescent” titre would provide adequate sensitivity and specificity for the diagnosis of novel H1N1 swine flu after the fact ?
Thanks much! Can you call me 408.353.2014-when you can.
Fantastic!!! I have a job innterview tomorrow and this has been SO helpful!!!
Do you happen to know of any FDA approved commercially available kit for the estimation of H1N1 IgG antibodies in human serum?
Do you happen to know of any FDA approved commercially available kit for the estimation of
IgG antibodies in human serum
I talked to my local health dept- they are to work on it but there doesn't seem to be one yet as of a couple weeks ago. Focus has been all about getting the vaccine out and delivered. All the best.
Amazing information, very useful for the understanding of this concept, thank you!
Pingback: Protection against 2009 influenza H1N1 by immunization with 1918-like and classical swine viruses
Thnx 4 d valuable info
I read your post with immense interest.
Since the CDC (or FDA) uses HI antibody titers as a measure for evaluating a vaccine for approval, your post implies that only vaccines that induce antibodies targeting the receptor binding region can be approved by CDC/FDA. Is this true? Recently, several broadly neutralizing antibodies were discovered (F10, CR6261). However, these antibodies target the membrane proximal region of HA. So does this mean that they can only be used as therapeutic antibodies?
Can you also post the guidelines that the CDC/FDA use in order to approve a vaccine? Also, could you provide the relevant information in the case of a drug approval?
Thank you for this useful information!
As you showed that HA assay is a good way to get the approximate titer of hemagglutinin domain-contained virus such as Influenza. However, the serotype of anti-flu antibody should fully recognized H and N; even the sera can inhibit the HA in assay, it still can't give the information regarding the specificity to N. So, HI assay can preliminarily screen the H-positive serum but not N-serotype. Is it right?
That is correct, the HI assay will not provide information on the
presence of antibodies against the viral neuraminidase (NA) protein.
Those antibodies can be identified by using a neuramindase-inhibition
(NI) assay. The NA assay measures the ability of the enzyme to release
sialic acids from a substrate; and the NI assay measures inhibition of
the NA by antibodies in serum.
For determination of viral titers, serial dilutions of influenza virus were ….. Comparison.