
The genetic material of retroviruses is RNA, but during infection it is converted to DNA which then integrates into the chromosome of the cell. If the infected cell happens to be a germ cell, then the viral DNA, now called called an endogenous retrovirus, becomes a permanent part of the animal and its offspring. One of our endogenous retroviruses, called HERV-H, infected human ancestors about 25 million years ago. HERV-H has been found to be important for the properties of human embryonic stem cells.
Embryonic stem cells (ES cells), which are derived from the inner cell mass of a blastocyst (which forms 4-5 days after implantation), are pluripotent – they can differentiate into every cell type in the human body. Being pluripotent means expressing a very different set of genes compared with somatic cells – the cells of skin, muscle, organs, to name a few. The genes that are expressed in ES cells are controlled by a small number of key proteins that regulate mRNA synthesis. If these proteins – just four – are produced in a differentiated cell, it will turn into an ES cell – an induced, pluripotent embryonic stem cell, or iPSC. This observation garnered Shinya Yamanaka the Nobel Prize in 2012.
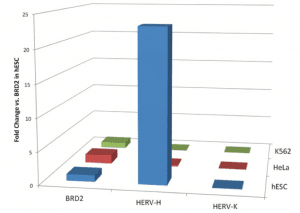
The first clue that HERV-H might be important for the pluripotency of ES cells was the finding that this DNA is preferentially expressed in human ES cells (the figure [credit] shows the expression of HERV-H in ES and two other cell types). When the levels of HERV-H RNAs are reduced (by RNA interference) in ES cells, the morphology of the cells changes – they become fibroblast-like, a sign of differentiation. In contrast, when fibroblasts are reprogrammed to become iPSCs, the levels of HERV-H RNAs rise. These findings suggest that HERV-H is essential for keeping ES cells pluripotent, and for making somatic cells pluripotent.
The HERV-H DNA in our genome is flanked by viral sequences called long terminal repeats, or LTRs. These provide initiation sites for the synthesis of viral mRNAs. In human ES cells the HERV-H LTRs appear to be enhancing the transcription of nearby human genes that are important for maintaing pluripotency. In an interesting twist, the HERV-H viral RNA is important for this activity: it appears to bind proteins involved in the regulation of mRNAs important for pluripotency. This observation explains why reducing HERV-H viral RNA leads to loss of pluripotency.
The HERV-H RNA made in human ES cells is not translated into protein because it contains many mutations that have accumulated over the past 25 million years. Therefore HERV-H is a long, non-coding RNA (lncRNA), a relatively recently discovered class of regulatory RNAs. There are about 35,000 lncRNAs in human cells that are involved in controlling a variety of processes such as splicing, translation and epigenetic modifications. Now we know that endogenous retroviruses can also produce lncRNAs.
Without endogenous retroviruses, humans might not be recognizable as the Homo sapiens that today walk the Earth. They might also be egg-layers – but the eggs would be white. Viruses don’t just make us sick.

The figure is from “HERV-H RNA is abundant in human embryonic stem cells and a precise marker for pluripotency”, Santoni F, Guerra J, Luban J, 2012 -http://www.retrovirology.com/content/9/1/111
If you click on the figure, it leads directly to the article citation. This is my way of citing figures taken from elsewhere.
Ok, I didn’t realize it. Thank you
Pingback: Why do we fight science? - Page 431 - US Message Board - Political Discussion Forum
Pingback: Ùيروساتنا البشرية؛ أربعون مليون عام من الصنع المستمر. | عرب Ùيس
Pingback: Ùيروساتنا البشرية؛ أربعون مليون عام من الصنع المستمر. | الÙضائيون
Pingback: Did viruses enable sex?
Pingback: viruses | [Veterinary and Medical Sciences
Pingback: Did viruses enable sex? – Virology