by Gertrud U. Rey

According to established scientific dogma, infection of cells with HIV-1 leads to delivery of the viral capsid into the cell cytoplasm, followed by “uncoating” of the capsid to release the single-stranded RNA genome. A viral-encoded enzyme called reverse transcriptase then catalyzes the conversion of the viral RNA into a single strand of DNA, which is copied to produce a double-stranded DNA molecule that subsequently enters the nucleus and integrates into the host genome. A number of recent studies have challenged this order of events, suggesting that capsid uncoating and reverse transcription of the viral RNA genome may actually occur inside the nucleus.
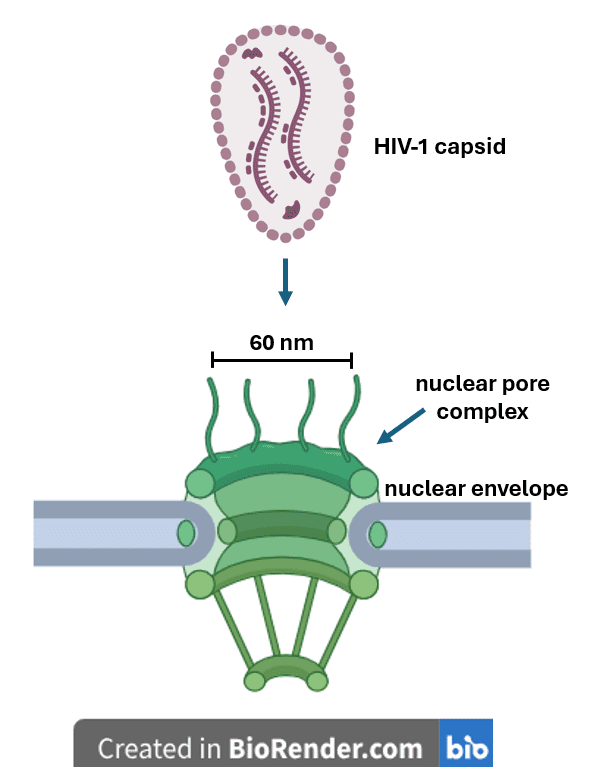
Any molecules that enter the cell nucleus must presumably pass through the nuclear pore complex – a series of channels in the nuclear envelope that collectively act like a sieve to only allow passage of certain particles. Large molecules can only traverse the complex if they are escorted by nuclear transport proteins known as importins. The widely held view that the HIV-1 capsid disassembles in the cytoplasm is based partly on the assumption that HIV-1 capsids cannot enter the nucleus because they are too big to pass through any of the nuclear pores. However, the details associated with HIV-1 entry into the nucleus are still poorly characterized and are thus the subject of ongoing research efforts.
A short report published in Nature Microbiology in 2020 showed that seemingly intact HIV-1 capsids do enter the nucleus, even when nuclear pores are blocked. The authors infected cells with HIV-1 and then added a protein to the cells that binds to the nuclear pore complex and expands to obstruct the passage of materials. When they monitored the trafficking of fluorescently labeled HIV-1 capsids by fluorescence microscopy, they found that a small number of fluorescent particles localized to the interior of the nucleus, despite the nuclear pore blockade. This observation suggested that HIV-1 may be able to enter the nucleus through alternative nuclear import pathways. The authors also showed that when they added an inhibitor of reverse transcriptase to the infected cells, HIV-1 capsids still made it into the nucleus, suggesting that nuclear import of HIV-1 capsids can occur independently of, and before, reverse transcription. In other words, uncoating of the HIV-1 capsid and reverse transcription of the viral RNA don’t necessarily have to happen in the cytoplasm, or before entry of HIV-1 into the nucleus.
Using a technique called cryo-electron tomography, the authors of a more recent study showed that the nuclear pores are actually larger than originally thought. They appear to be around 60 nanometers in diameter – just wide enough to accommodate the passage of an HIV-1 capsid. Electron microscopy analysis also revealed that when HIV-1-like capsids were added to cells that were depleted of nuclear transport proteins, the capsids still localized to the nuclear pore complex, suggesting that HIV-1 capsid is capable of targeting and binding to the nucleus even when importin proteins are absent. Using an assay that detects specific protein-protein interactions, the authors further confirmed that HIV-1 capsids interacted directly with proteins in the nuclear pore complex. They also showed that the capsids could transport cargo (i.e., a red fluorescent protein) across the pores and into the nucleus, while unencapsulated control red fluorescent protein remained outside the nucleus. These observations suggest that the HIV-1 capsid acts like a nuclear transport protein in that it directly catalyzes traversal of cargo across the nuclear pore; and that the capsid enclosure may protect the viral genome from cytoplasmic antiviral substances until it is safely inside the nucleus. One limitation of this study is that instead of using actual RNA-filled HIV-1 capsids, the investigators resorted to capsid-like particles having a similar size and shape as HIV-1, likely because they are easier and safer to handle. If authentic HIV-1 capsids do indeed mimic nuclear transport proteins, the interaction between the capsid and the nuclear pores could potentially be targeted with appropriate drugs to disrupt the viral replication cycle, as an alternative to existing HIV therapeutics.
These findings re-emphasize the fact that getting clear answers to a question requires repeated, disparate, and unbiased inquiry. Because science is a fluid process, concepts that are viewed as accepted dogma should be continually re-evaluated as new technologies and techniques emerge.
[The material in this blog post is also covered in episode 59 of my YouTube series Catch This.]

Well said.
Vero.
Até a descoberta da reverse transcriptasepor Temin e Baltimore o Dogma central da biologia era DNA RNA proteína.
Depois tudo mudou.
I am very happy to visit your blog. Now I have found what I want. Thanks a lot for sharing a piece of wonderful information which I have been looking for for a long period.
kitboga-phone-number
ROT
I really liked this article. Not just because of the elegant description of the experiments that led to this update about HIV infection but the final thought, Science is not fixed but a study of life’s incredible capacity to adapt to different situations. Our intelligence is constantly challenged and how often, scientists beat the odds. Really great reading.