COVID-19 vaccine candidates are garnering all the news these days, which is appropriate as they are our key to ending this pandemic. Earlier in this outbreak antiviral drugs received a good deal of attention, but they have proven less useful in curtailing infection. Less discussed are the many antiviral drug candidates that are in testing, including one that appears to be effective in a ferret model of infection.
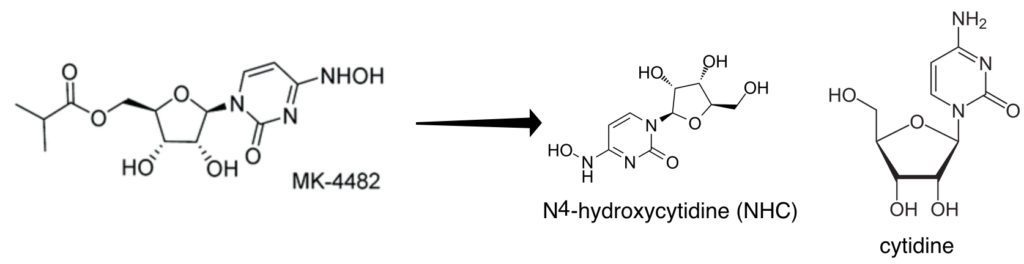

MK-4482 is an orally available pro-drug of the nucleoside analog N4-hydroxycytidine (NHC) (pictured above). The latter is a nucleoside analogue which is incorporated into RNA by the viral RNA-dependent RNA polymerase. Once incorporated into RNA, NHC is recognized as either C or U by the RNA polymerase. As a consequence, many mutations are introduced into the viral genome, causing lethal mutagenesis and inhibition of infectivity. NHC has been previously shown to have broad-spectrum anti-RNA virus activity and blocks transmission of influenza virus in a guinea pig model of infection.
When ferrets are inoculated intransally with SARS-CoV-2, the virus reproduces efficiently in the upper and lower respiratory tract. Infectious virus is detectable in nasal wash up to 7 days post-infection. The animals lose weight but do not succumb to disease. Oral administration of MK-4482 at 12 or 36 hours after infection, followed by treatment twice daily for 3.5 days, leads to complete clearance of infectious virus 24 – 36 hours later, demonstrating oral efficacy of therapeutic administration of the drug in ferrets.
To assess whether MK-4482 can impact transmission of SARS-CoV-2, animals were inoculated intranasally with virus and then co-housed with naive animals 30 hours later for 3 days. In experiments with untreated animals, all co-housed ferrets became infected. In contrast, when ferrets were treated with MK-4482 12 hours after infection, they did not transmit infection to co-housed animals, demonstrating that NHC can block transmission when given 12 hours after infection.
These results are promising and justify studies of the efficacy of MK-4482 in humans. The drug has already passed phase I safety trials which revealed that the drug reaches concentrations in the blood that exceed the antiviral level needed for inhibition of SARS-CoV-2 in cell culture. Consequently efficacy trials in humans appear to be warranted.
My one main concern with this study is that the drug was only administered to ferrets 12 and 36 hours after infection. It would be important to know how long after infection the drug can be administered and still limit infection and transmission. If, like the influenza antivirals Tamiflu and Relenza, the drug must be administered 24-48 hours after infection to have an effect on disease outcome, the usefulness might be limited.
With COVID-19 vaccines advancing in many countries, does it still make sense to pursue clinical development of antiviral compounds? The answer is yes. Antiviral drugs might be used to stop outbreaks and prevent transmission. For example, if an outbreak is detected in a nursing home, all occupants could be treated to prevent spread of infection. Indeed, if compounds such as MK-4482 had been available in December 2019, it might have been possible to impede the spread of SARS-CoV-2 before it left China.

The article is great thank you.
A broad spectrum anti-RNA-viral! Nothing like a pandemic to kick slow moving pharmaceutical development in the butt. Let’s get it done in time for the next pandemic.
My friend reports that Merck licensed it and they haven’t heard anything about it since that happened.