In “Influenza virus attachment to cells” we left the intact virion on the cell surface. The next step is that the viral genetic information – for influenza virus, the individual RNA segments – must enter the cell so that it can be reproduced. The mechanism for influenza virus, illustrated below, involves a step that is a target of the antiviral adamantanes.
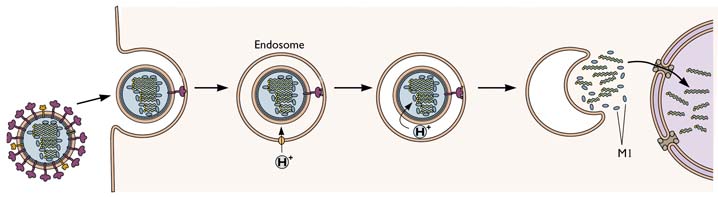

The entry of influenza virus into cells is one of the best undertood of all known viral entry mechanisms. After the virion attaches to sialic-acid containing receptors at the cell surface, the virus-receptor complex is taken into cells by endocytosis, a process by which cells normally take up molecules from the extracellular fluid. As the endosomal vesicles that contain the virus particles move towards the cell nucleus, their pH drops. This change in pH is accomplished by a cellular channel that pumps protons (H+) into the vesicle. When the endosomal pH reaches 5.0, the viral HA protein undergoes a conformational rearrangement. This change exposes a fusion peptide on the HA – a short, hydrophobic sequence that inserts into the endosomal membrane and causes it to fuse with the viral envelope. When this occurs, the viral RNAs are released into the cytoplasm. They are then transported into the cell nucleus where viral RNA replication occurs.
In the influenza virion, the viral RNAs are not naked, but bound to a number of viral proteins, including the M1 protein. This protein forms the shell that underlies the lipid membrane of the virion. Unfortunately, if the viral RNAs are bound to M1 protein when they are released from the virion, they cannot enter the nucleus. To get around this problem, the influenza virion has a few copies in its membrane of a protein called M2. This viral protein forms a channel in the membrane that actively pumps protons from the endosome into the interior of the virion. These protons lower the pH in the interior of the virion, releasing the viral RNAs from M1. In this way the RNAs can enter the nucleus.
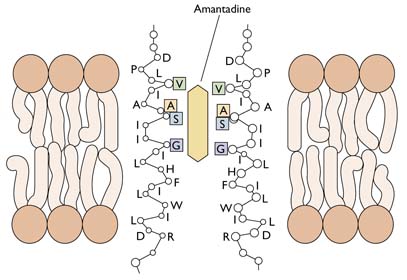
The M2 ion channel is the target of the antiviral adamantanes, depicted below. These compounds clog the channel and prevent it from pumping protons into the virion. In the presence of adamantanes, viral RNAs remain bound to M1 and cannot enter the nucleus. Therefore viral replication is inhibited. Resistance to adamantanes occurs by changes in amino acids that line the M2 channel. These changes prevent the drug from plugging the channel.


OK, a few questions: How do the viral RNAs make it into the nucleus?
And, learning about how amantadine and oseltamivir work make me wonder about side effects…why do side effects come about? Is it from something happening when the drugs are doing their job, so to speak, like actually clogging the ion channel, or is it something that happens on the way to that channel? or is it something else in the drugs – maybe some added, like a preservative?
And, lastly, how do we know all this? How do scientists figure these really complex processes out?
Okay, sorry about the endless questions…thanks!
Viral RNAs are transported into the nucleus through a pore-complex, shown in the illustration. This so-called nuclear pore complex is used by the cell to actively more large assemblies in and out of the nucleus. Proteins have to have very specific amino acid sequences to be transported through these pores; such sequences are called nuclear localization signals (NLS). Influenza viral RNAs are bound to a viral protein, NP, which has an NLS and therefore hijacks this import system.
Side effects of drugs occur because the drugs inhibit a cellular target. For example, in the case of amantadine, the drug may block a cellular pore, or might even inhibit a completely different kind of protein. The NA inhibitors might in the same way inhibit other proteins – they look like sialic acids, which could potentially interfere with many cellular processes. It's usually not something in the antiviral that is added.
Scientists figure out complex processes by using a model system, and then breaking down the process into small steps. Then they propose a hypothesis and do experiments to test it. Let's take nuclear import of influenza RNA. People wondered how the RNA could get into the nucleus because it doesn't have an NLS. So they proposed that one of the viral proteins bound to the RNA had an NLS. This NLS was found on the NP protein. Then the sequence was changed which prevented the NP from entering the nucleus. These experiments were done in cells in culture.
When I teach virology, I always teach not only the models, but how the models were obtained. I can't do that here because of time/space limitations. Anyone can teach facts. Not everyone can teach how the facts were obtained. If you learn how these processes are sorted out, then you will learn how science is done.
If the NP protein has an NLS then is the NLS specific to eukaryotes? Or is it more specific, like mammals? Not all NLS signals are created equally…
With amantadine, would the side effect be the interaction of the porins or with importin?
Are there any new M2 or NA inhibitors in the development?
Last question…Has any work been done on drugs that target the NP protein to either cleave the NLS or change the confirmation such that importin doesn't recognize the NLS?
Pingback: Mao Asada » ESCRT
This whole discussion seems very interesting to me since is one of my research areas, although I am not an experimentalist and I'm kind of new in the field. I have a few comments and questions I'd like to add.
As far as I know, there is a difference between an ion channel and an ion pump. The former acts according to a gradient, while the later can go against this gradient, therefore it needs to get energy from somewhere (eg ATP).
“As the endosomal vesicles that contain the virus particles move towards the cell nucleus, their pH drops. This change in pH is accomplished by a cellular channel that pumps protons (H+) into the vesicle.” I imagine this is a proton pump, not a channel, the same that takes protons out of the cell, using ATP found in the cell interior, only now it pushes them into the endosomal vesicle.
When the endosomal membrane fuses with the viral lipid envelope, the capsid, formed by many copies of M1, should be exposed now. ” To get around this problem, the influenza virion has a few copies in its membrane of a protein called M2. This viral protein forms a channel in the membrane that actively pumps protons from the endosome into the interior of the virion.” From this description it seems that M2 is actually a proton pump, using ATP from the cell to work? Do we know if this happens before, after or while the fusion is taking place? From this illustration (https://virology.ws/wp-content/uploads/2009/…), it appears as if M2 traverses both the viral lipid envelope AND the capsid, but from the illustration above this is not clear. So, where do the protons pumped by M2 end up, is it inside the capsid? Could this explain why the capsid disassembles? Do we know the 3D structure of M2 and the Amantadine?
Thanks!
Good article and great Q's and A's too.
The NP NLS has only been tested in mammalian systems. It is a 'nonclassical' NLS because it does not resemble classical import sequences.
Amantadine has neurological side effects due to its dopaminergic and adrenergic activity, and to its activity as an anticholinergic.
Yes, there are new NA inhibitors in development – see https://virology.ws/2009/01/28/new-influenza… and the reference therein.
As for drugs that target the NP NLS…no, none have focused on that step. It's a difficult assay for drug screening and others (NA, polymerase) are much easier to adopt to a high throughput format.
You are correct, the M2 forms a proton pump in the viral membrane. It does not traverse the underlying shell composed of the M1 protein. Yet the protons end up in the virion interior, where they dissociate the RNA-protein complex from M1, allowing dissociation of the shell formed by M1 (technically it is not a capsid). It is believed that the protons enter the virion before membrane fusion occurs, to prime the viral RNA for release into the cytoplasm. Yes, the structure of M2 and amantadine are known – see http://bit.ly/O0cJE.
Pingback: Influenza viral RNA synthesis
thanks for clearing that up, and the link, very helpful. I'm looking forward to read the following posts on the subject!
I am also curious about what you said not being technically a capsid. Copies of M1 form a shell encapsulating the genetic material inside the lipid envelope. Is the structure of this shell similar to those capsids of other non-enveloped spherical viruses in terms of symmetric arrangements? or is it more of a 'loose' structure (weaker inter subunit interactions)? Can you define a T number for example?
Thanks again for all your answers!
Pingback: Debate in science… « The Virelegy
Pingback: Ascription is an Anathema to any Enthusiasm › Searching the Alternate Routes
The M1-shell doesn't have any symmetry (e.g. icosahedral, found in
many capsids) nor can a T number be defined.
Sorry to go back to it but just to be clear are there two lots of ion pumping going on? (PS yes I know this is not a body building web site).
On first reading I took there to be an decrease in ph being created between the inside of the endosome relative to the cytoplasm
“When the endosomal pH reaches 5.0, the viral HA protein undergoes a conformational rearrangement. This change exposes a fusion peptide on the HA – a short, hydrophobic sequence that inserts into the endosomal membrane and causes it to fuse with the viral envelope. When this occurs, the viral RNAs are released into the cytoplasm.”
Which lead to the fusion peptide ‘bursting’ the the endosome. Does this release the vRNA or just release the intact viron? If the pump is in the endosomal wall why is it there, is the reduction of ph to 5.0 normal for an endosome during transport to the nucleus?
If there is only one pump in this story – M2 – then it is pumping H+ into the virus’ interior which causes the HA to re-jig its tertiary structure to expose the fusion peptide which breaks the endosome and frees the polymerase complex from the M1.
PS where does HA0 cleavage by the host protease fit in to all this.
PPS Thanks for the time you have taken to run explain all this.
I have a slightly offtopic question regarding M1. I have studied influenza A viruses and have a basic understanding of what role plays each protein, and how they work.
However it seems M1 is everywhere and nowhere all at once – intervening in many steps of the viral infection as well as affecting the virion structure, but still lacking a very definite role. It almost appears as if it was some sort of all-purpose molecular glue. So I'm left wondering how this protein actually functions? Is it a sort of molecule that is specific to influenza, or rather something to be found wildly in nature?
Thanks a lot for exposing and sharing your knowledge, it's extremely interesting!
Cyril
Pingback: Reassortment of the influenza virus genome
M1 protein is multifunctional, at least in terms of influenza virus
replication. It has a structural role in the virion, and also a role
in entry. Exactly how it functions won't be known until its structure
is determined. There are similar proteins in other enveloped RNA
viruses which have structural roles. None have yet been shown to
function during entry as does influenza M1.
Great Teaching. Here's my question: how many influenza virions does a typical hijacked cell eventually bud out?
Do each of those (or what %) then hijack new epithelial cells?
Thanks,
Steve
The yield of influenza virus would depend upon the cell type, but
typically about 1000 particles, of which ~100 are infectious.
Thanks so much herr professor. I've been trying to get the information you've shared for quite some time specificly for influenza. First- I have seen 500 for cocksackieviruses (Vernon Knight- Viral and Mycoplasmal infections of the Respiratory tract 1973 page 155) but nothing in the same book for influenza. I'd love to get a reference from you so I can read more about this.
I am interested in multiplication in epithelial cells as that is what flu loves to munch in our respiratory tract.
Next-I had no idea that only 10% of budded virions were viable. Is that typical for most virions? You called them particles. What is the difference?
You rule,
Steve
How do the imported RNAs transported to the nucleus?
Oops sorry, I see that question is addressed below.
Hi Am I right in thinking that the (swine flu) Novel H1 N1 RNA is made up of aproximatly 8 or so influenza viruses and not just swine influenza?? if so can you tell me which ones I am a student nurse and trying to do a presentation on the swine flu virus and although I can remember reading somewhere about Swine influenza virus containing 8 or more influenza viruses I cant remember where or which ??
thanks Karla Ward
Hey Karla,
All inflenza virions have 8 RNA strands within them. The RNA within the swine flu is from human, pig and avian flu virions. At the beginning of this thread you can see the intact flu virion at the left. Its hard to see the 8 RNA strands until the virion releases them in the target healthy cell's cytoplasm in the next to last illustration. In the last illustration at the right side, the RNA strands “are then transported into the cell nucleus where viral RNA replication occurs.”
Hi Am I right in thinking that the (swine flu) Novel H1 N1 RNA is made up of aproximatly 8 or so influenza viruses and not just swine influenza?? if so can you tell me which ones I am a student nurse and trying to do a presentation on the swine flu virus and although I can remember reading somewhere about Swine influenza virus containing 8 or more influenza viruses I cant remember where or which ??
thanks Karla Ward
Hey Karla,
All inflenza virions have 8 RNA strands within them. The RNA within the swine flu is from human, pig and avian flu virions. At the beginning of this thread you can see the intact flu virion at the left. Its hard to see the 8 RNA strands until the virion releases them in the target healthy cell's cytoplasm in the next to last illustration. In the last illustration at the right side, the RNA strands “are then transported into the cell nucleus where viral RNA replication occurs.”
Hi:
I have a trouble in finding a TEM photo about endosome of influenza. I saw the webpage” Release of influenza viral RNAs into cells” and also that diagram. I wonder if someone have seen the endosome by TEM? I cannot find the paper with this. Do you have this or these paper on hand?
Thanks.
Kevin
I found one paper published in 1981 “Infectious Entry Pathway of Influenza Virus in a Canine
Kidney Cell Line”. Very good paper to elucidate how the virus go inside cells.
I found that proteases of cell are available in the Golgi, Where the HA is trimmed, after glycosylation in rough endoplasmic reticulum(?). If the proper proteases would be available into Golgi, HA0 would be cleaved in cleavage site and then assemble as an INFECTIVE virion and embedded from host cell.
In the case of low pathogenic avian influenza (LPAI) viruses, HA0 can be cleaved with trypsin-like proteases, found in restricted anatomical sites, such as respiratory and intestinal epithelial cells, which accounts for the restricted replication and lower virulence. But for highly pathogenic avian influenza (HPAI) viruses, trypsin-like proteases or furin-like proteases can cleave HA0, furin proteases are present in many cells throughout the body, which causes higher virulence. In brief, the cleavage of the HA protein into the HA1 and HA2 proteins is essential for the virus to be infectious and produce multiple replication cycles.
Commonly, LPAI viruses are not infectious when are released from the host cell with an uncleaved HA protein. Because when they enter to the next host cell as endosome, FUSION would not be occurred. However in some cases, concurrent infection with bacteria that secreted proteases which cleaved HA of LPAI viruses, exacerbates the LPAI virus infection.
Quick question how does the Influenza virus obtain energy?
It uses the host cells own energy before that it is in a dormant like state till it enters the host cell
Why do influenza viruses have to enter the
nucleus to replicate
Before entry the virus is inert it needs the living cell tissue to become active and replicate
Pingback: FLU……. – www.drhelpdesk.in