by Gertrud U. Rey

Public health officials are continuing to monitor the spread of avian H5N1, the strain of influenza virus associated with “bird flu.” Although there is still no evidence that this virus can transmit from one person to another, two recently infected individuals are attracting close attention because the source of their infection is unclear.
One of the individuals is a child in California – the first documented pediatric case of H5N1 in the United States. The child allegedly had no exposure to any infected animals, and the California Department of Public Health is still investigating the possible origin of the virus. Contact tracing has not revealed any other H5N1 cases in the child’s social circle, suggesting that the virus was not acquired from or passed to another human. Fortunately, the illness was mild and the child is recovering well.
Meanwhile, a more serious H5N1 infection was reported in a British Columbian teenager, who is now in critical but stable condition. As with the child in California, it is unclear how the teen became infected; however, recent genetic sequencing analysis implies a possible exposure to wild birds. None of the teenager’s close contacts were infected with H5N1, which makes person-to-person transmission unlikely and indicates that the virus is not circulating in the community. The teenager’s symptoms started with an eye infection but later developed into a severe lung infection, suggesting that the virus evolved an enhanced ability to enter respiratory tract cells within that individual.
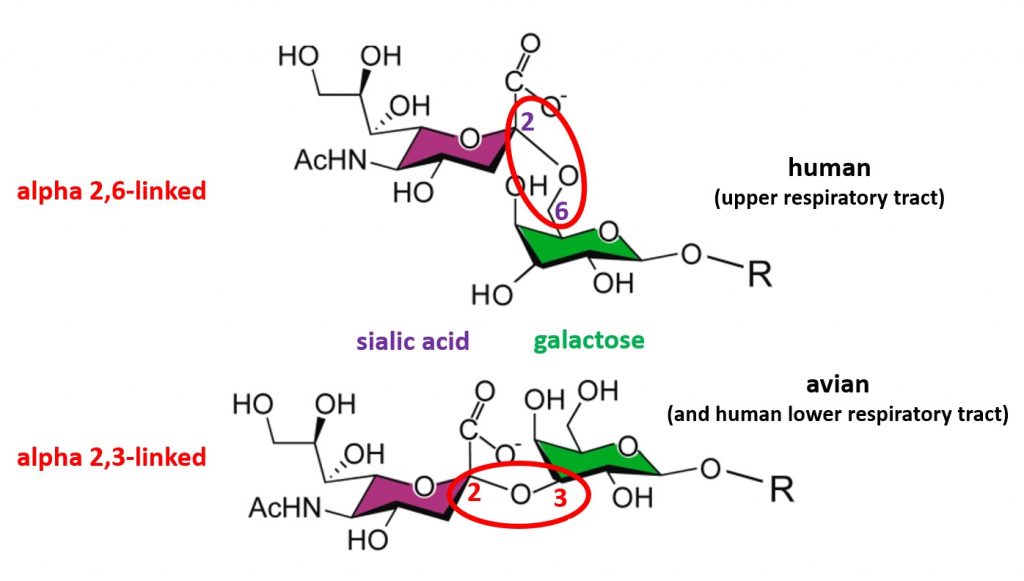
Influenza viruses enter host cells when the viral surface hemagglutinin protein (i.e., the “H” in H5N1) binds a host cell surface protein known as a receptor. Human influenza viruses bind receptors that consist of a sialic acid molecule linked to galactose via an alpha 2,6 glycosidic bond (top of image), and these receptors are located mostly on cells of the upper respiratory tract. In contrast, avian influenza viruses (including H5N1) preferentially bind sialic acids with an alpha 2,3 linkage (bottom of image), which are abundant on cells of the avian digestive tract and cells of the human lower respiratory tract. As mentioned in a previous post, this receptor preference prevents H5N1 from transmitting efficiently from one person to another. To pass easily among humans, H5N1 would at the very least have to acquire an amino acid change that allows it to bind sialic acid receptors with an alpha 2,6 galactose linkage.
A recent study, which analyzed all possible amino acid changes in the influenza virus hemagglutinin that affect alpha 2,6-linked sialic acid usage and cell entry, revealed that some mutations led to enhanced entry of viruses into cells that displayed alpha 2,6-linked sialic acid receptors. In particular, mutations that changed the amino acid glutamate at position 190 of the hemagglutinin protein to a glutamine or alanine, or mutations that changed the amino acid glutamine at position 226 to a leucine or arginine, led to enhanced entry of viruses into cells that had alpha 2,6-linked sialic acid receptors.
The published sequence of the H5N1 virus isolated from the British Columbian teenager displays nucleotide differences that produce ambiguous amino acid changes at positions 190 and 226 of the hemagglutinin amino acid sequence. The ambiguity is likely due to the presence of mixed viral sequences and further supports the notion that the virus evolved after it infected the teenager. Although these findings have inspired multiple alarmist news headlines, there are a couple of reasons for why there is currently no need to panic.
First, there are at least two other obstacles that prevent sustained human-to-human transmission of H5N1, in addition to receptor preference. One obstacle involves the H5N1 polymerase enzyme, which normally functions at 40℃, the temperature of the avian digestive tract, but would have to adapt to function at the 33-35℃ temperature range of the human upper respiratory tract to replicate in a human, in order to transmit effectively from this site. Another obstacle relates to the pH inside the endosome – the membrane vesicle that forms around a viral particle once it enters a host cell. H5N1 viruses are only stable in the high pH environment of the avian endosome and would easily degrade at the low pH of the human endosome, leading to inefficient replication and transmission to a new host.
Second, scientists have been anticipating an H5N1 pandemic for many decades. There are several effective FDA-approved antiviral drugs that are readily available, and a few experimental vaccines are also in development.
The changes observed in the sequence of viral RNA isolated from the teenager in British Columbia are necessary but not sufficient to trigger a human pandemic. Therefore, the public health risk associated with H5N1 influenza virus is still low at this time. Nevertheless, circulating viruses should be continually monitored for changes in genomic sequences that signal the potential for human-to-human transmission. Meanwhile, it is a good idea to avoid touching sick or dead animals in the wild, and to prevent contact between domesticated and wild animals.
[The material in this blog post is also covered in Catch This Episode 62. The receptor-binding preference of avian and human influenza viruses is explained very well in this episode of MedCram.]

thank you for this! sending this to my non-scientist friends who are getting scared by the fear mongering headlines coming out lately.
Sending this to my friends! This information helps clear the air about the headlines coming out about avian H5N1.
It seems to me that the fact we have 2,3 receptors on our eyes is another big barrier to a human pandemic. People with Bird Flu get extreme eye irritation and bleeding. If you can’t see, you also can’t mingle with other people well enough to spread viruses. Even variants that attack both types of receptors (as the cattle outbreak seems to) have this problem. A human pandemic requires that carriers have symptoms that aren’t severe enough to prevent normal functioning, in order to sustain uncontrolled spread.