by Gertrud U. Rey


Recent news headlines have been highlighting the global spread of H5N1, the strain of influenza virus that is typically associated with “bird flu.” This outbreak is the largest in recorded history, involving at least 50 million dead birds and countless non-human mammals, including sea lions, otters, mink, foxes, cats, dogs, and skunks. But what does this mean for us?
Although the virus has so far infected about 1,000 people worldwide, most of these infections have been in individuals who had direct contact with infected birds, and thus, the infections likely originated from those animals. There is currently no evidence to suggest that H5N1 can transmit efficiently from one person to another, a factor that is critical for triggering a human pandemic. As far as we know, there are several obstacles that prevent sustained human-to-human transmission of H5N1.
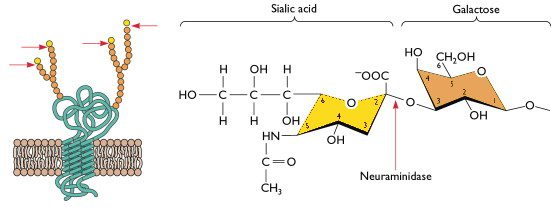
The first obstacle has to do with the host cell surface receptor that mediates viral entry for influenza virus infection in humans. To enter a cell, human influenza viruses bind receptors that consist of a sialic acid molecule linked to galactose via an alpha 2,6 glycosidic bond, and these receptors are located mostly on cells of the upper respiratory tract. In contrast, avian influenza viruses (including H5N1) preferentially bind sialic acids with an alpha 2,3 linkage (illustrated), which are abundant on cells of the avian digestive tract and cells of the human lower respiratory tract. Although H5N1 can infect and replicate in cells of the lower respiratory tract, its transmission to other humans from the lower respiratory tract is very inefficient, meaning that H5N1-infected people typically do not pass the virus on to others. In other words, because H5N1 cannot efficiently replicate in the upper respiratory tract, it doesn’t typically transmit among humans. In order for H5N1 to pass easily from one person to another, it would at the very least need to acquire an amino acid change that allows it to bind a sialic acid receptor with an alpha 2,6 galactose linkage.

A second obstacle that prevents sustained human-to human transmission of H5N1 influenza virus involves the H5N1 polymerase enzyme, which is responsible for replicating the viral genome. To function properly, this enzyme needs to be in an environment with a temperature of approximately 40℃ – the average temperature of the avian digestive tract. Because the human upper respiratory tract has a temperature range of 33-35℃, the H5N1 polymerase would need to adapt to function in this temperature range in order for H5N1 to replicate and transmit more effectively from this site.
A third obstacle relates to the pH inside the membrane vesicle that forms around a viral particle once it enters a host cell. This vesicle, called an “endosome,” transports the viral particle through the cytoplasm until the viral and endosomal membranes fuse to allow the viral RNA to enter the host cell cytoplasm. Human-adapted influenza viruses undergo this membrane fusion most efficiently in the low pH conditions of the endosomes of human cells. However, H5N1 viruses require a much higher pH for fusion, meaning that they could easily degrade in the low pH environment of the human endosome, and thus not produce an effective infection that could transmit virus to other humans.
The segmented structure of the influenza virus genome allows for frequent reassortment between segments, such that if a host cell is co-infected with two different strains of influenza virus, the segments can reassort to produce new virus strains. Pigs are susceptible to infection by both avian and human influenza viruses, making them likely mixing vessels for such reassortment events. Reassortment between the “right” influenza virus genes could lead to a new version of H5N1 that could infect and transmit from cells of the human upper respiratory tract, thus triggering efficient human-to-human transmission and a potential pandemic.
Fortunately, many scientists are preparing for such a scenario by developing H5N1-specific diagnostic tools, antiviral drugs, and vaccines. For example, virologist Scott Hensley and colleagues have generated a highly promising monovalent mRNA vaccine that completely matches the currently circulating strain of H5N1. So far, the vaccine produces great antibody responses in mice, but it still needs to be tested in ferrets, and then obviously, humans. There are also many other research groups working on both H5N1-specific and multivalent influenza virus vaccines directed against all known influenza virus subtypes.
There is no way to predict if and when H5N1 will evolve so it can pass easily between humans, although the probability for such a phenomenon is as real as the recent emergence of SARS-CoV-2. Considering that the mortality rate from H5N1 infection in humans is estimated to be higher than 50%* (compared to less than 1% from SARS-CoV-2), the consequences of a potential H5N1 pandemic would be a lot worse than those of the present pandemic. The fact that H5N1 already appears to transmit fairly well between non-human mammals as evidenced by an outbreak on a Spanish mink farm is highly concerning. The current H5N1 outbreak warrants increased surveillance and preparedness, and it further highlights the importance of a comprehensive One Health approach for detecting and controlling pandemic threats.
*This estimate by the World Health Organization likely does not take into account the total number of infections, which is probably much higher than we think. A higher number of total infections would decrease the rate of mortality.
[A big thank you to Joanna Pulit-Penaloza for the useful discussions, which helped me clarify some of the concepts in this post. The material in this blog post is also covered in Catch This Episode 45.]

Comments are closed.