Molnupiravir might be the first highly effective antiviral drug given emergency use authorization for treatment of COVID-19. Should we be concerned about the results of a recent study which show that the drug is mutagenic in cells?
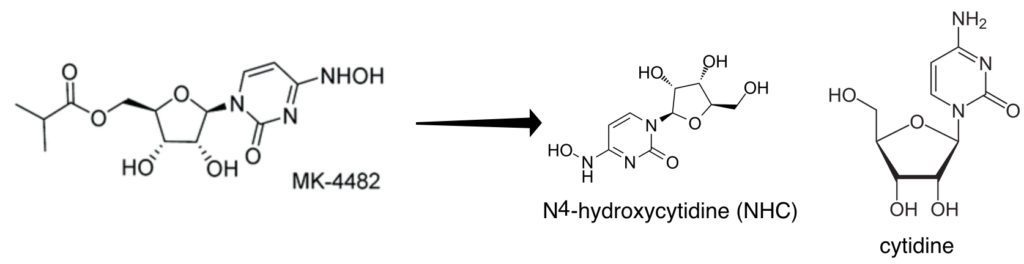

Molnupiravir is an orally available pro-drug of the nucleoside analog N4-hydroxycytidine (NHC). The latter is a nucleoside analogue which is incorporated into RNA by the viral RNA-dependent RNA polymerase (pictured above). Once incorporated into RNA, NHC is recognized as either C or U by the RNA polymerase. As a consequence, many mutations are introduced into the viral genome, causing lethal mutagenesis and inhibition of infectivity. NHC has been previously shown to have broad-spectrum anti-RNA virus activity and blocks transmission of influenza virus in a guinea pig model of infection. It has been shown to block SARS-CoV-2 transmission in ferrets and results of a phase 2/3 clinical trial look promising, leading to a request for emergency use authorization.
N4-hydroxycytidine could be metabolized by the host to produce the 2′-deoxyribonucleotide form, which could be incorporated into cellular DNA and lead to mutagenesis. To test this hypothesis, a mutagenesis assay was used in Chinese hamster ovary cells (CHO-K1). These cells have one copy of the gene encoding the enzyme hypoxanthine phosphoribosyltransferase (HPRT), which makes the cells sensitive to the base analog 6-thioguanine (6-TG). If NHC were mutagenic, changes in the HPRT gene would allow cells to survive in the presence of 6-TG.
Cells were exposed to NHC for 32 days and assayed for sensitivity to 6-TG. The drug conferred 6-TG resistance in a dose-dependent manner. Two other antivirals that are base analogs, ribavirin and favipiravir, displayed either no or modest mutagenic activity in this assay. Sequence analysis of HPRT mRNA revealed the presence of base changes.
Molnupiravir is a far more active coronavirus antiviral than favipiravir and ribavirin, yet NHC has the distinct ability of causing mutations in cell DNA. The concern is that such mutations could lead to cancer or birth defects in a developing fetus. Whether or not Molnupiravir might cause cancer in humans is not known. However Merck, the developer of Molnupiravir, is required to carry out a series of gene toxicity studies before phase I testing of the compound in humans. Included is the Ames test, which uses bacteria to assess mutagenic activity of a compound. Bacteria do have the enzyme which can convert NHC to the DNA form. The results of these safety studies will not be published until after the drug receives EUA, but presumably nothing was observed that would preclude clinical trials.
Consequently until we have further information about preclinical studies on NHC, we should be cautious in our interpretation of the results of mutagenesis assays in CHO cells.

Very interesting. Thank you for this.
Dear Sir;
Is this looklike thalidomide drug effect for baby in longterm?
Thank you very much for your courtesy and consideration.
Sincerely yours;
Charoen Hanpanjakit
This very same drug was not authorized a couple of years back it was actually knocked back. The senator in charge was moved on and now the drug it is back alive and the same. I was interested in the U TUBE presentation Miracle Treatment or Harmful Drug by MD. DR Christy Risinger and her thoughts on this exact drug I think it is a must watch for those who care. Have a good day Everyone
Thanks, Vincent. Very helpful to me in my labors on molnupiravir today.
p.s. It’s been a hydoxychloroquine-remdesivir-ivermectin-molnupiravir past few days. Oy. Glad to have your clear language in the post.
Hydoxychloroquine-remdesivir-ivermectin-molnupiravir, the list grows but still no perfect candidate COVID-19 drug. Hydroxychloroquine and ivermectin must be used prophylactically, which is hard to do with SARS-CoV-2, remdesivir isn’t very effective, and molnupiravir is mutagenic to the host. How about trying CsCl? – it is unlikely to be mutagenic, it can be used at any time in the disease course, and it is already FDA approved for clinical use. “Take a scant tsp of CsCl mixed in a glass of juice, wait 6-8 hrs, then eat a banana. Expect diarrhea. Repeat 3-4 times.” What could be simpler? The only problem is that it has never been tested against plus-strand RNA viruses.
Pingback: New Antivirals: Could They Be Game Changers? - GVN
What mutations would it cause in DNA code/chemistry