by Gertrud U. Rey

Antiviral agents are a class of drugs that inhibit the replication of viruses at various stages of their replication cycle. One class of antivirals is small molecules known as “chain terminators,” because their addition to the end of a chain of DNA or RNA prevents the addition of any subsequent nucleotides, thus ending the replication of DNA or RNA.
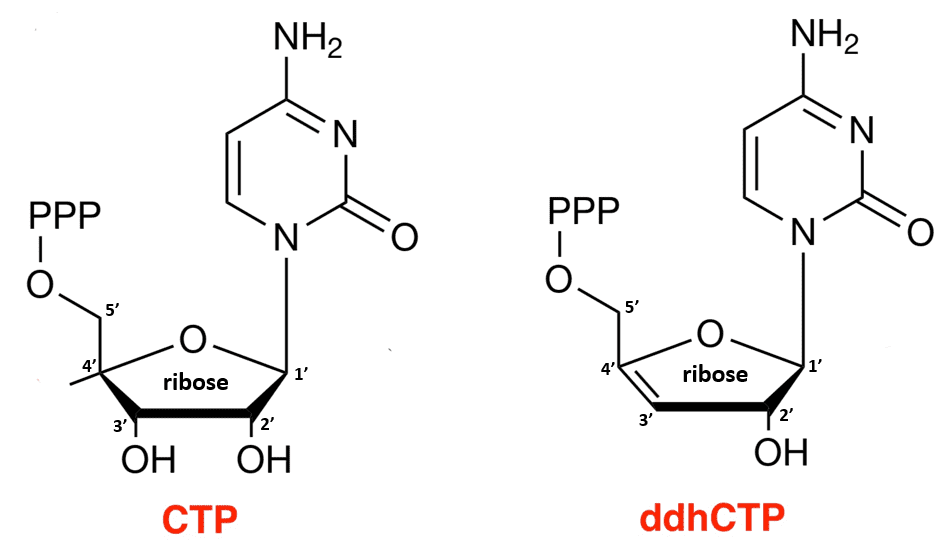
Individual RNA nucleotides consist of a ribose sugar that is connected to one of four different bases (adenine, guanine, cytosine, or uracil) via its 1′ carbon and a group of three phosphates via its 5′ carbon. For example, the ribose in the nucleotide cytidine triphosphate (CTP) is connected to a triphosphate group and a cytosine base (left side of Figure). Addition of this CTP to the end of a replicating RNA strand results in loss of two of its phosphates and connection of the remaining phosphate to the 3′ hydroxyl (OH) of the ribose in the preceding nucleotide in the strand. The absence of a hydroxyl at the 3′ carbon of a nucleotide ribose (right side of Figure) would prevent the binding of a subsequent nucleotide and would thus make that nucleotide the last one in the chain.
Viral infection in humans induces production of interferon, which further stimulates the expression of other genes, one of which is viperin (virus inhibitory protein, endoplasmic reticulum-associated, interferon inducible). Research in recent years has shown that viperin catalyzes the conversion of CTP to 3′-deoxy-3′,4′-didehydro(ddh)-cytidine triphosphate (ddhCTP) (right side of Figure). As the “deoxy” portion of its name implies, ddhCTP lacks a hydroxyl at the 3′ carbon of its ribose sugar, making it a chain terminator. In human cells, the conversion of CTP to ddhCTP by viperin leads to broad antiviral activity against various viruses, including human cytomegaloviruses, West Nile virus, dengue virus, hepatitis C virus, and HIV, without any toxic effects on host DNA replication.
Some bacteria and archaea encode genes with notable sequence similarity to vertebrate viperins; however, until recently, their role was unclear. Because of the antiviral role of viperins in human cells, the authors of a recent publication aimed to determine whether prokaryotic viperins participate in defenses against viruses that infect bacteria, known as bacteriophages (or, simply “phages”). Knowing that genes encoding antiviral defense proteins tend to cluster together in the prokaryotic genome, the authors searched for the presence of such clusters in a variety of bacterial and archaeal genomes. They found that although most clusters of viperin-like genes did not colocalize with defense genes, 60% of the genes in one cluster were located near genes encoding CRISPR-Cas systems, restriction enzymes, and other bacterial defense genes.
To see whether the prokaryotic viperin-like genes in these clusters encoded proteins with antiviral activity (pVips), the authors cloned 59 of these pVip genes into E. coli under the control of an inducible promoter, allowing their expression to be switched on or off. Infection of these pVip-producing E. coli with a range of phages revealed that about half of the tested pVips had inhibitory activity against the phages. Most of the pVips inhibited bacteriophage T7, but some of them also inhibited P1, lambda, SECphi6 and SECphi18 phages, without any toxic effects on the bacterial host cells. Surprisingly, when human viperin was expressed in E. coli under the same conditions, it protected against infection by T7 phages, suggesting that the antiviral functions of viperin and viperin-like gene products are highly conserved.
The authors next wanted to determine whether pVips catalyze production of ddhCTP, like human viperins. Assuming that small molecule fragments extracted from pVip-producing E. coli having anti-phage activity would include modified nucleotides, the authors analyzed E. coli lysates by mass spectrometry, a technique that reveals the chemical identity of molecules and other chemical compounds. They then compared the small molecules identified from cells producing pVips to molecules obtained from E. coli containing human viperin. This analysis revealed that cells producing some prokaryotic viperin-like molecules contained derivatives of CTP, including ddhCTP, while others did not, suggesting that only some viperins catalyze the production of ddhCTP. However, some prokaryotic viperins seemed to produce modified ddh derivatives of other nucleotides, like GTP and UTP. Collectively, these results suggest that prokaryotic viperins produce antiviral nucleotides, just like human viperins.
Although environmental bacteria have long been used to identify novel antibiotics, the notion of extracting antivirals from bacteria is new. Nevertheless, many of the antiviral drugs used in clinical settings are chain terminators, including acyclovir for herpes virus, AZT for HIV, and sofosbuvir for treating hepatitis C virus infections. The present study may have revealed an additional source of diverse antiviral molecules that could be adapted for clinical treatment of human viral infections.
[The study described in this article was also discussed at length on TWiM 233. The material in this blog post is also covered in this video.]
