
The results from a phase I clinical trial to test the safety and immunogenicity of a universal flu vaccine candidate reported encouraging results €“ strong titers of broad and functional antibodies persisted for over a year in healthy adults following vaccination.
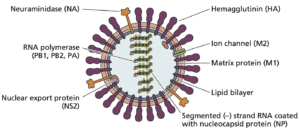
Influenza viruses contain segmented RNA genomes. The viral envelope contains two types glycoproteins or ‘spikes’ that facilitate viral entry into host cells €“ hemagglutinin (HA) and neuraminidase (NA). Typically, HA and NA proteins stud the viral envelope at a ratio of four to one. Additionally, there are three types of influenza virus €“ A, B, and C. Influenza A viruses are further characterized by subtype based on their HA and NA proteins. Three HA subtypes (H1, H2, H3) and two NA subtypes (N1, N2) have be shown to cause widespread influenza transmission in humans.
The HA molecule contains two structural regions: the head and stalk. Spatially, the head is more prominent than the stalk, and antibodies to the head of the HA molecule have been shown to neutralize viral infectivity. The structural and functional characteristics of influenza viruses allow for antigenic drift and antigenic shift. Antigenic drift results when influenza virus strains develop frequent amino acid changes in the head domain, and the strain eventually evolves into one that can no longer be neutralized by antibodies to the parent virus. Antigenic shift occurs when an influenza A virus acquires the HA domain (and potentially the NA domain) from a different viral subtype.
The combination of these phenomena requires that flu vaccines be reassessed each year based on the viral strains currently circulating in the human population. These vaccines are efficacious when they are well matched to the circulating strains; however, mismatches are not uncommon. As such, vaccinologists have been seeking to develop a ‘universal’ flu vaccine that would confer protection against all seasonal, zoonotic, and emerging pandemic influenza viruses.
Current seasonal influenza vaccines primarily target the HA head domain of the circulating strains. However, as mentioned previously, the head domain can escape neutralization by accumulating sufficient mutations through antigenic drift. The HA stalk domain, however, is more conserved; therefore, researchers hypothesized that a vaccine targeting the HA stalk domain might offer protection independent of antigenic drift or shift.
To provoke an immune response to the HA stalk domain, researchers at Mount Sinai developed a sequential vaccine strategy by generating chimeric HA (cHA) proteins consisting of conserved stalk and head domains from various avian influenza subtypes. Most adults already possess immune memory to the H1 HA domain as well as antibodies and memory B cells specific to the stalk domain; therefore, it was proposed that vaccinating individuals with cHA constructs that consist of head domains from different avian influenza virus subtypes and a conserved stalk domain might redirect the immune response to the stalk.
The clinical trial consisted of 5 different treatment groups:
Group 1: LAIV8-IIV5/AS03
Day 1: Intranasal (i.n.) live-attenuated influenza virus (LAIV) vaccine expressing ch8/1 HA and an N1 NA.
Day 85: Intramuscular (i.m.) inactivated influenza virus (IIV) vaccine expressing ch5/1 HA and an N1 NA with an adjuvant (AS03).
Group 2: LAIV8-IIV5
Day 1: (i.n.) LAIV vaccine expressing ch8/1 HA and an N1 NA.
Day 85: (i.m.) IIV vaccine expressing ch5/1 HA and an N1 NA with no adjuvant.
Group 3: Placebo Control 1
Day 1: (i.n.) Saline solution.
Day 85: (i.m.) PBS
Group 4: IIV8/AS03-IIV5/AS03
Day 1: (i.m.) IIV vaccine expressing cH8/1N1 with AS03.
Day 85: (i.m.) IIV vaccine expressing cH5/1N1 with AS03.
Group 5: Placebo Control 2
Day 1: (i.m.) PBS
Day 85: (i.m.) PBS
The regimen for Groups 1 and 3 comprised live-attenuated influenza virus (LAIV) followed by an inactivated influenza virus (IIV). This combination was tested based on previous studies showing that it had provoked optimal antibody responses with influenza virus vaccines in humans and non-human primates and with chimeric HA-based vaccines in ferrets. This combination had also been found to convey better protection against infection than the chimeric HA-based IIV-IIV regimen in ferrets; therefore, it was hypothesized that the intranasal LAIV followed by the intramuscular IIV boost might confer better protection than the two intramuscular doses of IIV (Group 4).
Ultimately, the study reported that Group 1 participants did not produce significant anti-stalk antibody titers after the day 1 LAIV dose; however, when these participants were boosted with IIV5/AS03 they induced a strong anti-stalk antibody response. When the boost was given without an adjuvant in Group 2, lower anti-stalk antibody titers were observed. In Group 4, however, the initial administration of IIV8/AS03 induced a very strong anti-stalk antibody response. Although these titers dropped slightly between days 25 and 85, they increased again after the administration of IIV5-AS03 on day 85. Further, these antibody titers persisted above baseline levels at 420 days after vaccine administration. While Groups 1 and 2 antibodies also persisted, they did so at lower levels. Anti-stalk antibodies did not increase in the placebo groups over the course of the study, and no significant adverse reactions to the vaccine were reported. Additionally, mice treated with post vaccination serum were protected from viral challenge as compared to mice treated with pre-vaccination serum. Although one might have predicted Groups 1 and 2 to show better protection than Group 4 based on the data established in ferrets, animal models do not always approximate vaccine responses in humans.Though encouraging, these results are still preliminary, and future trials will shed light on whether antibodies to the stalk protein will prove to be as protective as those elicited by natural infection. It may take several years to develop the diversity of chimeric hemagglutinins needed to make a universal flu vaccine, and later phase clinical trials to test the vaccine’s efficacy and superiority to existing vaccines would need to be conducted. This study does demonstrate, however, that €œyou can develop a vaccine strategy that produces stalk-reactive antibodies in humans,€ said virologist Florian Krammer in an interview for Science, one of the lead investigators on the project. It represents an important step towards developing a universal vaccine strategy that could replace the seasonal model.
Image credit: Principles of Virology, ASM Press
