by Gertrud U. Rey
Viruses are universally defined as “obligate intracellular parasites” because they cannot replicate outside of a host cell and depend on that cell and its various metabolic factors for replicating their genome. Based on this definition, most virologists agree that viruses are not alive.
When giant viruses were initially discovered, they were found to violate multiple principles of virology. For example, mimiviruses can be parasitized by small viruses called virophages that can only replicate if they confiscate the replication factors of a co-infecting mimivirus. Because the virophage also inactivates the mimivirus during this process, some interpret this scenario as a virus infecting another virus, a previously unheard-of phenomenon. In turn, mimiviruses have defense mechanisms that inhibit virophage replication, a property that is analogous to eukaryotic anti-viral interferon-mediated defenses. Additionally, mimiviruses encode proteins that participate in protein synthesis – another unusual property for a virus.
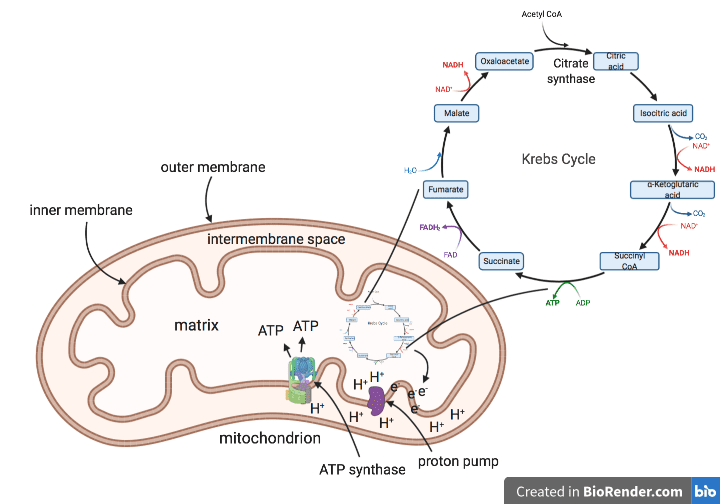
Some mimiviruses also have a gene that codes for citrate synthase, an enzyme involved in the Krebs cycle. The Krebs cycle is integral to cellular metabolism in living organisms because it ultimately powers the production of adenosine triphosphate (ATP), the cell’s molecular currency of energy. The cycle takes place in the matrix of the mitochondrion (pictured), where it feeds electrons into a string of complexes in the inner mitochondrial membrane known as an electron transport chain. As electrons move down this chain, they release energy, which is used by membrane-resident enzymes to pump protons from the matrix across the membrane into the intermembrane space (pictured as “proton pump”). This produces a concentration gradient, which is a difference in the concentration of protons on one side of the membrane compared to the other. To achieve equilibrium, the protons move back into the matrix through the action of another membrane resident enzyme called ATP synthase, which captures the energy of the protons to produce ATP. In other words, a concentration gradient across a membrane produces an electrical potential and is usually associated with the ability to generate energy in living cells.

Based on the knowledge that some mimiviruses encode a component of the Krebs cycle, a group in Marseille wanted to determine whether giant viruses can produce their own energy. To do this, they infected a species of amoeba, the natural host of giant viruses, with Pandoravirus massiliensis, a virus with the largest known viral genome encoding many proteins with unknown functions.
The authors isolated viral particles from P. massiliensis-infected amoebae and treated them with P. massiliensis-specific antibodies and a dye that detects electrical potential. This technique produced fluorescence in the membranes of P. massiliensis particles, indicating the presence of an electrical potential, in contrast to control virus particles isolated from cells infected with cowpoxvirus, which did not fluoresce. To confirm that the observed fluorescence represented a real concentration gradient with potential for electron transport, the authors treated the P. massiliensis particles with CCCP, a chemical that inhibits movement of electrons. This treatment led to diminished membrane fluorescence, suggesting that the observed membrane potential was real. Interestingly, the intensity of the electrical potential could be modified with addition of variable concentrations of acetyl-CoA, a known regulator of the Krebs cycle.
In an effort to determine how the P. massiliensis genome could play a role in energy metabolism, the authors did a sequence alignment with a database of conserved sequence domains known to be involved in energy metabolism. This revealed that P. massiliensis contains genes for nearly all enzymes in the Krebs cycle, but when these genes were cloned and expressed in bacterial cells, only one of them, isocitrate dehydrogenase, was functional. In agreement with this observation, the authors also found that mature P. massiliensis particles released from amoeba cells did not produce any ATP. Nevertheless, when amoeba cells were infected with P. massiliensis that were pre-treated with CCCP, they produced a lower number of viral particles, suggesting that the observed membrane potential might play a role during infection.
The authors conclude that these findings “position this virus as a form of life.” I disagree with this conclusion for the following reasons. Although P. massiliensis encodes numerous Krebs cycle enzymes, only one of them seems to be functional. Furthermore, P. massiliensis particles did not produce any ATP, meaning that this virus cannot produce its own energy. Even if it did, it still depends on the host cell for many other replication factors, including those needed to make proteins. As long as a virus requires a cell for replication, it is still a virus, and hence not alive.
Still, these findings are interesting and remind me of bacteriophage ϕKZ, a giant virus discussed in a previous post. After infecting a bacterial cell, ϕKZ assembles a nucleus-like shell, which shields the viral DNA from bacterial immune enzymes. Any discovery that reveals genes in viruses that suggest the potential for cell-like functions raises at least a couple of questions. Are these genes remnants of cellular genes, thereby suggesting that these viruses originated from ancient parasitic cells? Or did these giant viruses acquire the genes over time to gain more independence from host cells? Either way, pandoraviruses are aptly named because their study continues to yield surprising discoveries.
[A video covering the material of this blog post can be found here.]

Thanks for the interesting post. I am an EE by training with a background in scientific instrument design, but in the last few months have taken up self study of molecular biology motivated by curiosity about Covid originally, but now motivated by plain old general curiosity. Consequently lately I have drifted into territory already familiar to me, namely energy conversion, and bioenergetics has been a particular focus for the last few weeks. Therefore this post, and the work for the paper cited, is very timely for where I am at in my learning progession!
For the record (as much as my opinion matters) I agree with your conclusion that the evidence does not point to pandoraviruses being alive, but that nevertheless this is very compelling work.
Viruses are alive
Defining viruses as alive or not depends on a definition of alive. One of them is “being subject to darwinian evolution”. In this view, if viral particles (virions) are not alive, since they are survival and transmission forms, as bacterial spores or dry seeds, viral genomes reprogramming infected cells are obviously submitted to darwinian evolution, and the forces acting on them are completely different from those of the cells and organisms that they infect. Therefore considering their whole cycle viruses are fully alive. For translation and energy, some viruses indeed encode components of the translation machinery, but no virus encode for ribosomal RNA. Considering that most part of cell’s energy is devoted to protein synthesis, it make sense that viruses don’t produce their own energy and instead rely on cell’s energy and ribosomes for protein and particle production.