by Gertrud U. Rey

Throughout the current pandemic, there has been a lot of talk about T cells and their role in protecting against SARS-CoV-2 infection and disease. Some data suggest that 20-50% of people with no prior exposure to SARS-CoV-2 have T cells that recognize SARS-CoV-2 peptides, and that these T cells may be a result of recent infections with one or more of the seasonal human coronaviruses. However, it is unclear whether these “cross-reactive” T cells actually protect from SARS-CoV-2 infection and disease.
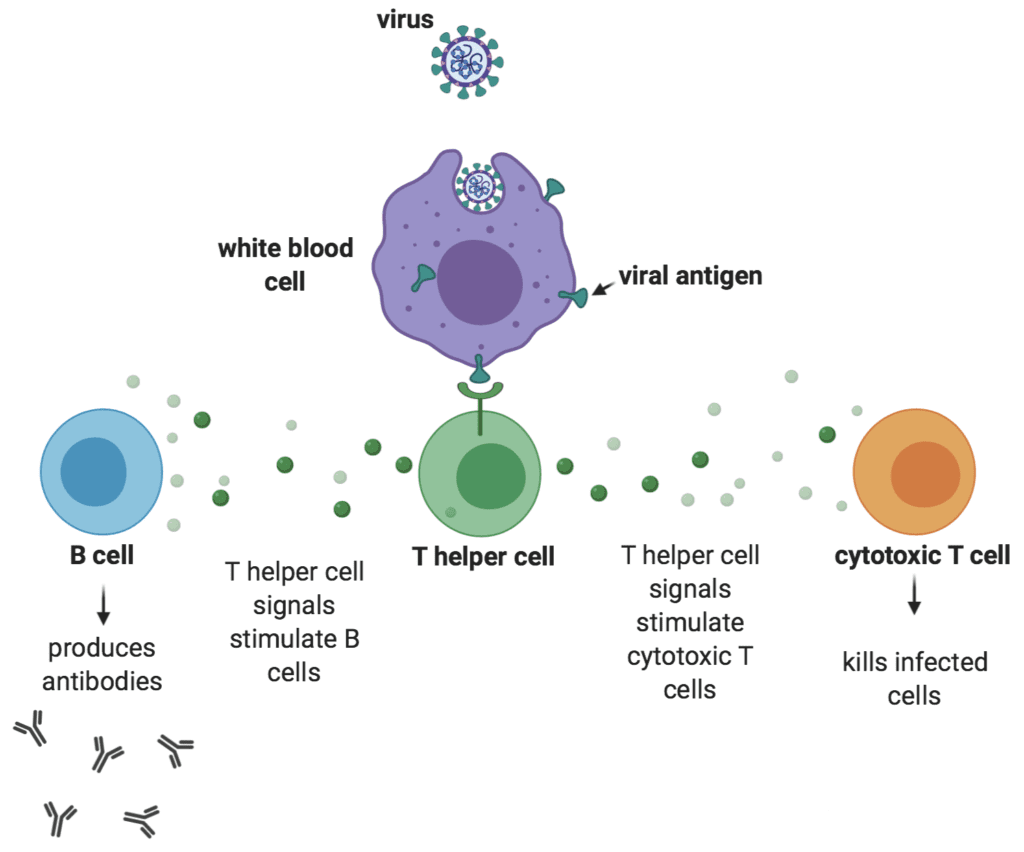
T cells are an important part of the adaptive immune response, which initiates during a first exposure to a pathogen and protects from re-infection and disease upon a second exposure to the same pathogen. During that first exposure, T helper cells sense the presence of one or more proteins (i.e., antigens) on the surface of the invading pathogen and release a variety of signals that ultimately stimulate B cells to secrete antibodies to those antigens, white blood cells to destroy ingested microbes, and cytotoxic T cells to directly kill infected target cells (see schematic). Before T cells encounter their first antigen, they are considered to be “naïve.” Upon their first contact with an antigen, they begin to mature and differentiate into either cytotoxic T cells or memory T cells. As we age and encounter more and more pathogens, the ratio of our memory T cells to our naïve T cells increases – a phenomenon sometimes referred to as “immunological age.”
Based on evidence from several labs, some have suggested that pre-existing cross-reactive memory T cells in people with no prior exposure to SARS-CoV-2 may have a protective effect. However, recent findings indicate that this may not be the case.
Several research groups from the U.S. and Australia analyzed blood samples from individuals with no prior SARS-CoV-2 exposure with the intent of better defining the range of T helper cells that can recognize antigenic portions known as epitopes in the SARS-CoV-2 genome. To ensure that the blood donors had never been infected with SARS-CoV-2, the researchers used stored samples that had been collected between 2015 and 2018. The authors found that the blood samples contained T cells that can recognize SARS-CoV-2 sequences that have at least 67% similarity to seasonal coronavirus sequences. However, the authors also found that people who had experienced a previous infection with SARS-CoV-2 had stronger and more specific (i.e., higher avidity) memory T cell responses to SARS-CoV-2 peptides than people with no prior exposure, and more than half of these responses were directed to epitopes in the spike protein. This suggests that SARS-CoV-2 memory T helper cells preferentially target viral proteins that are made in abundance during infection.
The authors conclude that an infection with a seasonal coronavirus may induce a range of memory T cells that have substantial cross-reactivity to SARS-CoV-2. However, they are careful to note that the clinical relevance of these data remains unclear and that there is no evidence that these memory T cells have any functional role in protecting from infection or disease.
Based on these findings, several groups of investigators in Germany wanted to determine whether the observed pre-existing T cell memory response in people with no prior SARS-CoV-2 exposure is protective against COVID-19. They first tested T cell activity in the blood of donors with and without prior exposure to SARS-CoV-2 by exposing their blood to highly immunogenic SARS-CoV-2 peptides. Blood from donors with a prior exposure contained memory T cells that recognized SARS-CoV-2 peptides very well, especially peptides derived from the spike, membrane, and nucleocapsid proteins. Blood from donors with no prior exposure also contained low levels of SARS-CoV-2-reactive memory T cells, but this reaction was more scattered and directed against multiple viral proteins.
To further characterize the pre-existing cross-reactive T cells in blood samples from people with no prior SARS-CoV-2 exposure, the authors compared the numbers of memory T cells and naïve T cells in the blood samples by analyzing them for the presence of protein markers that are characteristic for each type of cell. A substantial portion of SARS-CoV-2-reactive T cells from donors with no prior exposure were naïve T cells, whereas from COVID-19 patients most were mature memory T cells. Because memory T cells are more easily activated following an infection than naïve T cells, the authors speculate that deficient, low avidity memory T cells in people with no prior SARS-CoV-2 exposure may compete with naïve T cells and prevent their activation and maturation into highly specific memory T cells upon infection with SARS-CoV-2. This could potentially lead to an inferior immune response in people with no prior SARS-CoV-2 exposure.
Some have suggested that young patients and children may be particularly well protected from SARS-CoV-2 infection and/or disease because they are frequently infected with seasonal coronaviruses and thus presumably have high levels of pre-existing memory T cells. However, the authors found that compared to young people, older people with no prior SARS-CoV-2 exposure actually had higher numbers of SARS-CoV-2 cross-reactive memory T cells, but these T cells had a decreased avidity to SARS-CoV-2 epitopes compared to memory T cells from people with prior SARS-CoV-2 exposure.
A further comparison of T cell responses in patients with mild or severe COVID-19 revealed that although the latter had high numbers of SARS-CoV-2 specific T cells, these T cells had reduced target specificity and avidity compared to T cells from patients with moderate disease. The authors conclude that this unfocused response may result from recruitment of a broad range of pre-existing memory T cells in people with increased immunological age and may contribute to development of severe COVID-19 in the elderly.
The initial discovery of SARS-CoV-2-specific memory T cells in individuals with no prior exposure to SARS-CoV-2 had inspired the hypothesis that these T cells could possibly protect these individuals from disease and might partially explain why children are less susceptible to COVID-19. However, in light of these new findings it is likely that pre-existing SARS-CoV-2-specific memory T cells may contribute to the wide spectrum of disease severity among the general population and may actually be partially responsible for severe COVID-19 in the elderly.
[For an in-depth discussion of these two papers, I recommend TWiV 657 and Christian Drosten’s “Das Coronavirus Update,” episodes 58 and 60.]

2 questions occur to me:
How/what is it that the T helper cell sends to the B cell that lets it know what antibodies to make? Does it send parts of the antigen for the B cells to work with?
Have they thought to try the experiment the other way around? Would the SARS-CoV-2 infected persons’ specific T cells protect them from other coronaviruses–and even lead to a reduction in economic losses to the ‘common cold’?