by Gertrud U. Rey
Antibodies are large proteins that are made by B cells of the adaptive immune system. Most people think that antibodies function only as a whole molecule, but some of the individual fragments of an antibody can also bind and neutralize antigens. 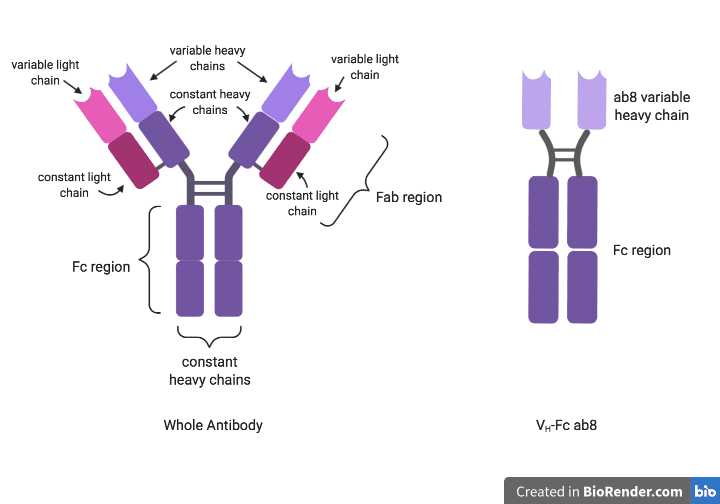

An antibody consists of two heavy chains and two light chains that assemble into a Y-shaped structure (left side of Figure). The stem of the Y is known as the “fragment crystallizable” (Fc) portion and is composed of two heavy chains. The two arms of the Y are known as the “fragment antigen-binding” (Fab) portions and are each composed of one heavy chain and one light chain. As its name suggests, the top half of each Fab fragment is the antigen-binding region of the antibody, and it is variable – meaning that it varies between antibodies that are produced by different B cells. The bottom half of each Fab fragment and the entire Fc region are constant, meaning that they are identical in all antibodies of the same isotype, but differ in antibodies of different isotypes. For example, the constant regions are identical in all IgG antibodies but differ between IgG and IgA antibodies.
In an effort to identify anti-SARS-CoV-2 antibodies suitable for preventing and treating SARS-CoV-2 infection, the authors of a recent publication screened 100 billion different anti-SARS-CoV-2 antibody candidates for their ability to bind and/or neutralize SARS-CoV-2. This eventually led to the discovery of “ab8,” an antibody fragment consisting of a variable heavy (VH) region and having particularly potent SARS-CoV-2 binding specificity and neutralization activity. To increase the binding avidity of ab8 (i.e., the stability of its interaction with an antigen) and extend its longevity in the human body, the authors fused this fragment to the Fc domain of human IgG1, an abundant and stable type of human antibody. This produced the molecule hereinafter referred to as “VH-Fc ab8″ (right side of Figure).
The authors found that VH-Fc ab8 can bind various conformations of the SARS-CoV-2 spike protein, including when the spike protein is bound to a cell surface. VH-Fc ab8 can also bind to and neutralize six different SARS-CoV-2 isolates having different amino acid changes in the receptor-binding domain, suggesting that it is broadly cross-reactive. Notably, it does not bind to human cells, meaning that it does not seem to interfere with normal cellular functions.
As a next step, the authors evaluated the ability of VH-Fc ab8 to prevent SARS-CoV-2 infection in mice. If given to mice before they were infected with SARS-CoV-2, VH-Fc ab8 inhibited viral replication at all doses tested, but it only neutralized virus at the highest dose of 36 mg/kg. Although these results were encouraging, it is often difficult to interpret data obtained in mice in terms of clinical relevance in humans, because mice don’t develop the COVID-19-related disease pathologies observed in humans. Hamsters more closely imitate human SARS-CoV-2 infection in the lung, suggesting that they could be a useful mammalian model for COVID-19. VH-Fc ab8 caused significantly reduced levels of infectious virus in the lung, nasal mucosa, and saliva of hamsters when administered one day before (i.e., “prophylactically”) or six hours after SARS-CoV-2 infection (i.e., “therapeutically”) compared to untreated control animals, suggesting that it could be used to both prevent and treat SARS-CoV-2 infection. Although VH-Fc ab8 led to greater reduction of virus levels when given prophylactically than when given therapeutically, therapeutic administration still led to significantly decreased viral loads in treated animals compared to untreated control animals, even at very low doses. VH-Fc ab8 not only alleviated pneumonia and reduced lung viral loads in hamsters, but it also reduced virus shedding in the upper airway, which could help with reducing transmission.
The authors also found that when they gave hamsters the same dose of either VH-Fc ab8 or IgG1 ab1 – a full-sized version of the antibody, and then examined their concentrations in the serum five days later, levels of VH-Fc ab8 were significantly higher than those of the full-sized antibody. This suggests that the systemic distribution of VH-Fc ab8 is more long-lived than that of a full-sized antibody.
Although small animal models can provide key insights into the pathogenic mechanisms of viral infections, they are often poor predictors of human disease outcomes. The therapeutic timeline followed in the hamster experiments (i.e., administration of VH-Fc ab8 six hours after infection) would also be difficult to reproduce in humans because therapeutic drugs are not usually administered until well after symptom onset. Therefore, it would be difficult to determine whether the therapeutic effect of VH-Fc ab8 observed in hamsters would be the same in humans.
That being said, there are clear advantages to using antibody fragments instead of whole antibodies. Their small size allows them to penetrate more efficiently to sites of infection and bind antigens more easily and with more specificity. Smaller molecules also diffuse more easily through tissues, meaning that they could be administered by routes other than injection, such as by inhalation. Furthermore, because the molecular weight of VH-Fc ab8 is only about half that of a full-sized antibody, smaller quantities would be needed to obtain the same number of molecules, meaning that antibody fragment therapeutics could be more easily mass-produced.
There is no question that we are in dire need of an effective therapeutic drug to treat SARS-CoV-2 infection. If the results observed in these animal experiments can be duplicated in humans, VH-Fc ab8 would be an attractive option for both treating and preventing SARS-CoV-2 infection.

Excellent explainer of important work!
I was surprised by the increased half-life of the Ab fragment. My naive assumption was that smaller stuff is more likely to be excreted from the kidneys. I’m curious if multimers of the small fragment would behave more like the full-length Ab because their respective molecular weights would be more similar.