
To diagnose someone who is infected with SARS-CoV-2, a long swab is inserted deep into the nasal sinus to provide samples of the nasopharyngeal mucosa, a site of virus reproduction. The material collected is then subjected to RT-PCR to determine the number of copies of viral RNA. This procedure is not ideal: it is difficult to do properly, and may cause extreme discomfort to the patient. The sampling could induce coughing or sneezing, placing the healthcare worker at risk. Comparing serial samples is unreliable because different quantities of mucus can be sampled each time.
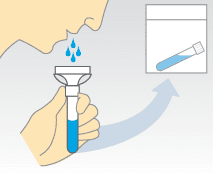
Saliva sampling has great appeal because it is not only non-invasive, but can provide consistent sample sizes and can be self administered (who hasn’t spit into a tube?). However we do not know whether assaying SARS-CoV-2 RNA in saliva is better than a nasopharyngeal swab.
To answer this question, the detection of SARS-CoV-2 RNA by nasopharyngeal swabs administered by healthcare workers was compared with self-collected saliva from 44 COVID-19 patients. Higher levels of viral RNA were found in saliva samples compared with nasopharyngeal swabs.
In another study of 22 participants administered multiple nasopharyngeal swabs or who provided multiple saliva samples, there were 5 cases in which a negative nasopharyngeal swab was followed by a positive result during the next collection. In contrast, there were no cases where saliva samples were first negative and then positive.
Finally, saliva was positive for SARS-CoV-2 in two healthcare workers who were asymptomatic and had tested negative by nasopharyngeal swab.
While these observations should be confirmed in a larger study, they suggest that saliva sampling for SARS-CoV-2 could be a sensitive, reproducible self-administered test for identifying both active and subclinical infections.
If the results of larger studies show that the saliva assay is a sensitive and reliable assay for detecting active infection with SARS-CoV-2, it could simplify testing in countries that lack nasopharyngeal swabs, personal protective equipment, and trained health care personnel.
If I were the COVID-19 Czar, I would have the saliva test sent to every individual in the US at the cost of the US government. Everyone would receive a kit consisting of a bar-coded tube into which they would spit, then record the barcode via cell phone to CDC. The tube of saliva would be mailed or dropped off at local collection boxes. The results of the RT-PCR assay would indicate who is currently infected in the US. This information will help to understand what fraction of the population might have immunity to infection.

Pingback: Spit for SARS-CoV-2 – Virology Hub
Funny typo: “This procedure is not ideal: it is difficult to do properly, and may cause extreme *comfort* to the patient.”
Thanks for your informative blog (including the Trial by Error series).
I agree, but hope you will rethink “record the barcode via cell phone to CDC”. Not every individual has a smartphone and as you say, testing has to include everyone. (Younger kids, older adults, groups like Amish, etc……)
Both the nasal swab and the spit tests rely on PCR methods that require manpower (or womanpower) and machinery, and are therefore unavoidably expensive. They are real-time assays, measuring relicating virus. Antibody tests can be performed on a drop of blood, like diabetes tests, and are much cheaper and less personnel-intensive, but are only meaningful after the immune system has had time to do its thing. We will likely see a proliferation of publications using both methods, but will not have a final answer until 80% of the population has been tested. After we have all this information, the virus will mutate to SARS-CoV-3.5 or something, and we can start making all the same mistakes all over again.
Astonishing that whoever designed the first test method didn’t think to try saliva (or even a shorter swab, or tonsil swab) in the first place!
All that rigmarole of health authorities fighting over swab supplies! :/