
The genome of vaccinia virus encodes 4 proteins for binding to cells, and 11 proteins for fusion. The latter form the entry fusion complex or EFC. A clue that these proteins are not evenly distributed came from the results of electron microscopy studies, which revealed that the sides of virus particles bind to cells, while fusion occurs at the tips.
Super-resolution microscopy and single particle analysis were used to show that the vaccinia virus binding proteins are located on the sides of virus particles, while EFC proteins are at the tips. This polarized pattern is diagrammed in the figure below (WT).
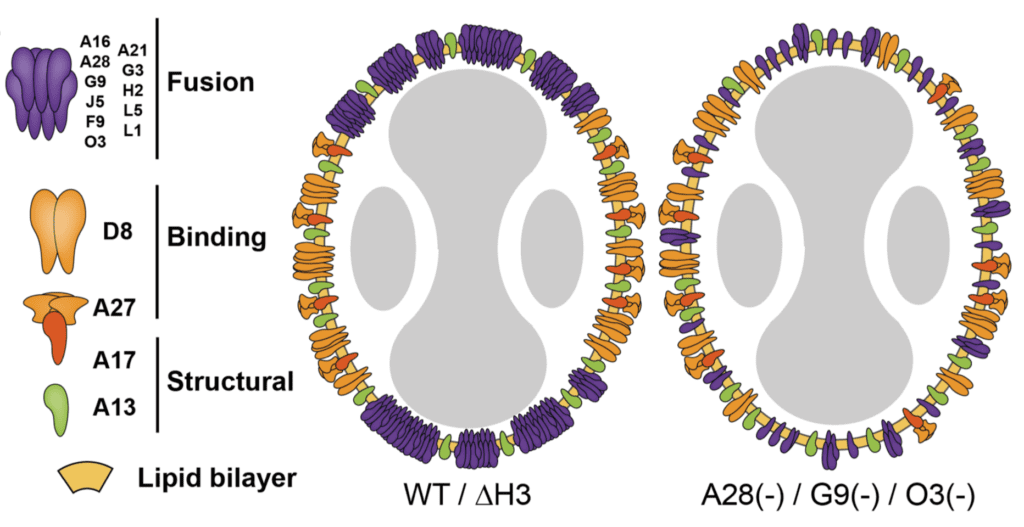

Removal of single genes encoding EFC components, such as G9 or O3, led to loss of this polarized structure (right panel in figure). These viral mutants are defective for fusion, suggesting a role for polarization in this virus entry.
Another viral protein, A27, is not a component of the EFC but is essential for fusion. Viruses lacking the A27 gene also display non-polarized glycoproteins, as shown in the figure below.
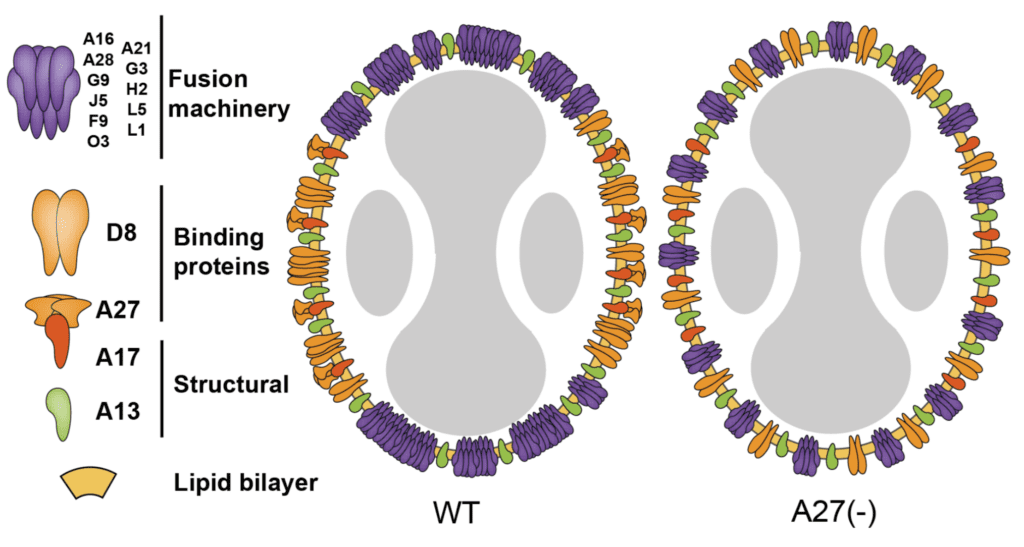

Although the A27 protein is not a transmembrane protein – it is attached to the virus surface via interactions with the A17 protein (see illustration above) – it nevertheless is required for polarized clustering of the EFC at the tips of virus particles.
The authors believe that the clustering of the vaccinia virus EFC is essential for efficient virus-cell fusion. What is the function of having fusion proteins localized to the tips of the virus particles? The authors suggest that concentrating the fusion proteins at the virus tips increases the chances of successful infection. Why this is not the case for most other viruses is a good question – or maybe it has simply not been examined.

Pingback: Viral glycoproteins are not always randomly distributed - Vetmedics
Pingback: Viral glycoproteins are not always randomly distributed – Virology