
There are over 500,000 endogenous retroviruses in the human genome, about 20 times more than human genes. They were acquired millions of years ago after retroviral infection. In this process, viral RNA is converted to DNA, which then integrates into cell DNA. If the retroviral infection takes place in the germ line, the integrated DNA may be passed on to offspring.
The most recent human retroviral infections leading to germ line integration took place with a subgroup of human endogenous retroviruses called HERVK(HML-2). The human genome contains ~90 copies of these viral genomes, which might have infected human ancestors as recently as 200,000 years ago. HERVs do not produce infectious virus: not only is the viral genome silenced – no mRNAs are produced – but they are littered with lethal mutations that have accumulated over time.
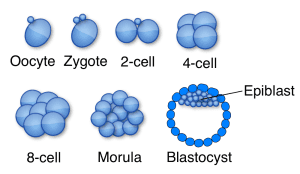
A recent study revealed that HERVK mRNAs are produced during normal human embryogenesis. Viral RNAs were detected beginning at the 8-cell stage, through epiblast cells in preimplantation embryos, until formation of embryonic stem cells (illustrated). At this point the production of HERVK mRNA ceases. Viral capsid protein was detected in blastocysts, and electron microscopy revealed the presence of virus-like particles similar to those found in reconstructed HERVK particles. These results indicate that retroviral proteins and particles are present during human development, up until implantation.
Retroviral particles in blastocysts are accompanied by induction of synthesis of an antiviral protein, IFITM1, that is known to block infection with a variety of viruses, including influenza virus. A HERVK protein known as Rec, produced in blastocysts, binds a variety of cell mRNAs and either increases or decreases their association with ribosomes.
Is there a function for HERVK expression during human embryogenesis? The authors speculate that modulation of the ribosome-binding activities of specific cell mRNAs by the viral Rec protein could influence aspects of early development. As Rec sequences are polymorphic in humans, the effects could even extend to individuals. In addition, HERVK induction of IFITM1 might conceivably protect embryos against infection with other viruses.
The maintenance of open reading frames in HERV genomes, over many years of evolution, suggests a functional role for these elements. Evidence for such function comes from the syncytin proteins, which are essential for placental development: the genes encoding these proteins originated from HERV glycoproteins. However, not all endogenous retroviruses are beneficial: a number of malignant diseases have been associated with HERV-K expression.

I recommend the work of Luis Villareal “Viruses and the Evolution of Life” and “Origin of Group Identity” He sees a crucial role for viruses in the development of life. One provocative finding (of many) is the homology between the “tube” between conjugating bacteria and the structure of bacteriophages through which viral genetic material is inserted into bacterial cells.
retroelements (transposons) or endogenous retroviruses? Is it justs “semantics”?
Retroelement is the name for a broad class DNAs in the genome (40% of the human genome) with sequences related to RT. This includes retrotransposons, which predate retroviruses, and endogenous retroviruses, which are the consequence of a retrovirus infection. HERVK are endogenous retroviruses, not retrotransposons. Steamer, which I discussed previously, is a retroelement, not an endogenous retrovirus, in the clam genome.
Pingback: Epigenetics vs the fossil record | RNA-Mediated
The Rec protein is generally considered to have similar functions as the HIV Rev protein. It is clear that the Rev protein, generally considered an RNA export factor ,also has functions at the cytoplasm at the level of mRNA translation. It is therefore really intriguing that this study suggests that Rec affects not only export, but also polysome association of some cellular mRNAs.
Pingback: Students Speak: Clemson University College of Health, Education, and Human Development | Clermont Country Internship
If you are interested in embryonic research,you can search http://www.creative-animodel.com/Animal-Model-Development/ES-cell-based-gene-overexpression.html for embryonic information related transgenic technology.
This may be a aspect of STEM CELL treatment.