
A sampling of the ratio of bacteriophages to bacteria in a variety of mucosal surfaces (sea anemone, hard and soft coral, polychaete, teleost, human gum, and mouse intestine) revealed higher ratios when compared to non-mucosal samples (e.g. neighboring sea water or saliva). A model bacteriophage of E. coli, T4, was used to show that phage specifically attach to mucus: adherence to cultured cells was reduced when mucus was not produced or removed by chemical treatment.
The principal macromolecules in mucus are mucin glycoproteins, which consist of a polypeptide chain linked to hundreds of variable, branched sugar molecules. Mucins are continuously produced at mucosal surfaces which gives rise to a thick protective layer. Phage T4 was found to attach specifically to mucins and not other components of mucus such as protein or DNA. The attachment of phage T4 to mucus-producing cultured cells reduced the number of bacteria that could attach to and kill the cells. This antimicrobial effect was substantially reduced when a strain of phage T4 was used that cannot lyse its bacterial host. Therefore phages bound in mucus protect cells by infecting and lysing bacterial invaders.
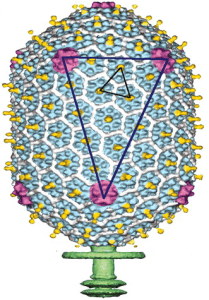
Phage T4 attaches to mucins through immunoglobulin-like (Ig-like) proteins present in the viral capsid. First discovered in antibody molecules, the Ig domain has since been found in hundreds of different proteins with various functions and appear to be encoded in ~25% of dsDNA phages. The Ig domain, typically 80 amino acids in length, is often involved in interactions with other proteins or ligands. For example, the Ig domains of antibodies interact with antigens, and the poliovirus receptor interacts with poliovirus via an Ig domain on the receptor molecule. The capsid of phage T4 contains 155 copies of an Ig-like protein called Hoc (colored yellow in the image). Deletion of the phage T4 hoc gene reduced binding of the virus to mucin, showing that adherence to mucin requires Ig-like protein domains.
These results demonstrate that a model bacteriophage, T4, attaches to mucus via an interaction between viral Ig-like capsid proteins and mucins. The ability of phages to attach to mucin clearly helps protect cultured cells from bacterial attachment and killing. Bacteriophages may be part of a previously unrecognized mucosal immune defense system. This suggests a symbiotic relationship between phages and metazoan hosts: the phages provide protection to mucosal surfaces, and in turn are provided hosts (bacteria) in which to reproduce. However, additional experiments are required to prove the authors’ conclusion of a “key role of the world’s most abundant biological entities in the metazoan immune system”. It will be necessary to directly demonstrate that phages attaching to mucins in mucus can protect an animal (e.g. mice) from bacterial invasion. This will not be an easy experiment because the phage and bacteria composition of mucus is likely to be complex and continuously changing as mucus is sloughed from cells and new mucins are produced.
The finding that phages play roles in mucosal immunity would have far-reaching consequences for human health. Some fascinating questions that come to mind include: do phage populations play roles in human diseases? Are they altered in human diseases and can we correct these diseases by restoring phage populations? Are the phage populations altered by antimicrobial therapy that alters bacterial populations? Do phages contribute to development of the immune system by modulating bacterial populations? Might mucus-bound phages stabilize the microbiome?
Update: Michael Schmidt and I discussed these remarkable findings on episode #59 of the science show This Week in Microbiology.

Pingback: TWiM #59: Are viruses part of our immune system? | This Week in Microbiology