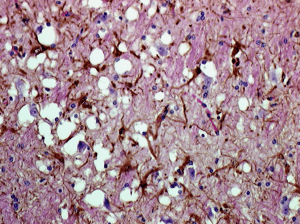

Prions are found in mammals and in fungi, but only in mammals are they infectious and pathogenic. All mammals make normal forms of the prion protein (PrPc) which is found in many tissues including the nervous system. The pathogenic form, called PrPSc, is a structurally altered form of PrPc. The PrPSc protein, named after the first prion disease studied, scrapie in sheep, causes PrPc to undergo a structural transformation to the pathogenic form. The PrPSc protein becomes deposited in amyloid fibrils in the brain, leading to neurodegenerative diseases known as transmissible spongiform encephalopathies (TSE), after the sponge-like appearance of the brain observed in afflicted animals (image).
There are three different ways to acquire a TSE. One is by infection: a human consumes meat that contains PrPSc, or receives a corneal transplant from a donor with an undiagnosed TSE . The PrPSc proteins make their way to the brain where they cause the host’s PrPc to misfold and become the pathogenic PrPSc. The more PrPSc that is made, the more the normal PrPc is converted to the pathogenic form. After an incubation period of many years, the host develops an invariably fatal neurodegenerative disease characterized by dementia in humans. There is also a familial form, in which mutations in the gene encoding PrPc are inherited; these cause the PrPc protein to misfold to form the pathogenic form. In the sporadic form PrPc spontaneously converts to PrPSc without any known mutation or infection.
TSEs occur in different forms with varied symptoms and pathology. There are TSEs of humans (Creutzfeld-Jacob disease, fatal familial insomnia, Gerstmann-Sträussler syndrome, Kuru) cows (bovine spongiform encephalopathy or mad cow disease), sheep and goats (scrapie), deer, elk, and moose (chronic wasting disease), and of a variety of other mammals.
This brings us back to the mad American cow, the first in the US since 2006. It died on a dairy farm and was tested for BSE as are 40,00o other cows each year in this country. The reason why this is big news is that back in the 1990s there was an outbreak of human TSE in the United Kingdom caused by consuming beef from animals with BSE. The cows acquired BSE by being fed processed animal byproducts as protein supplements, which unknowingly contained pathogenic prions. Bt the time the disease was detected in cows, contaminated meat had already entered the human food chain. Cows are routinely tested for BSE precisely to avoid a similar outbreak of human TSE.
The dead cow apparently had atypical BSE – that is, it was not a consequence of eating contaminated meat and it was not an inherited disease. Atypical BSE is caused by strains of prions distinct from other forms. This is good news because it means that the feed that the cow was receiving was not contaminated with pathogenic prions. Furthermore, the cow was not destined for meat production; it was a dairy cow that had died and was selected for random sampling.
Could the milk produced by this cow and consumed by humans pose a risk for transmission of a TSE to humans? It is known that ewes with scrapie shed infectious and pathogenic prions in their milk. However cows with BSE have much less PrPSc accumulation in peripheral tissues, and in particular lymphoid tissues which include the mammary glands. It seems unlikely that cow milk contains prions, but it is a question worth revisiting. Pathogenic prions are highly resistant to heat, ultraviolet irradiation and other extreme conditions, so would certainly survive the pasteurization process.

It’s probably also worth noting that milk is processed very differently from most meat, and the difference probably reduces the risk of prion transmission dramatically. When you buy a steak, you’re getting meat that came from a single animal, and even ground beef probably segregates into more or less single-animal batches because of the way it’s processed. Large-scale dairy operations, however, milk hundreds of cows at once, pooling the milk into huge vats for pasteurization and packaging.
If one sick cow contributes a dilute sample of PrpSc to the milk pool, the final product would have only vanishingly small quantities of the pathogenic protein in any given carton. That’s going to make it extremely difficult to test the milk for prions, but it should also make it pretty hard to get BSE from drinking milk.
How can they tell this was an atypical strain of the prion?
Different digestion patterns with proteinase K.
Aha, thanks. That was one thing I didn’t pick up from any of the reports.
Scrapie can be transmitted to sheep by grazing on the same grass that scrapie-infected sheep have previously been on. What are the risks (if any) of transmission of this atypical strain to other cows it lives with, or future cows reared in its environment?
Pingback: TWiV 181: ORFan poxviruses and nIRFing prions
Pingback: TWiV 181: ORFan poxviruses and nIRFing prions | Alan Dove, Ph.D.
I wouldn’t be so assured. All it takes is less than 1/4 tsp to infect MILLIONS. Also how to we really know just how many dairy cows have BSE that just haven’t shown symptoms yet. It lays dormant remember. Sorry but the milk, cheese, butter etc…can be just as infected as the muscle, brain and spinal cord. There are no assurances here, none!