
Viral infection of a healthy host usually leads to the production of both antibodies and lymphocytes. Antibodies generally bind to virus particles in the blood and at mucosal surfaces, blocking the spread of infection. In contrast, T-cells recognize and kill infected cells. The presence of specific antibodies has historically been used as an indicator of viral infection, partly due to the simplicity of the assay. In practice, dilutions of patient sera can be mixed with infectious virus to measure its ability to block infection (virus neutralization or hemaggultination-inhibition), or used to detect antibodies to viral proteins (ELISA or western blot).
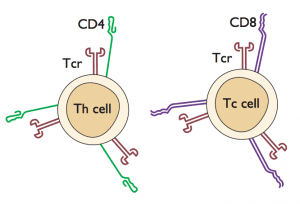
Another option for assessing previous viral infection is to determine whether virus-specific T-cells are present. In the past this type of assay has been difficult to carry out with large numbers of samples, but technical advances have now made it possible to do population screens for T-cell responses. In the current study, peripheral blood mononuclear cells (which contain T-lymphocytes and other blood cells) were obtained from patients and placed in culture. Next, pools of overlapping peptides that span most of the influenza viral proteins were added to the cells. These peptides were derived from influenza H5N1, H3N2, and H1N1 proteins. When T-cells recognize a peptide (via the T-cell receptor that recognizes the peptide presented by another cell type; see figure), the cells respond by producing interferon gamma, which can be readily measured.
The Vietnamese patients used for this study were part of a rural community where human and avian infections with influenza H5N1 had been previously documented. Twenty-four of of 747 individuals had evidence for the presence of T-cells that recognize peptides from the H5 HA more strongly than peptides from H3 or H1 HA. Another 111 samples had T-cells that react with H5, H1, and H3 peptides. If all positive patient samples (those lead to production of interferon gamma) are included, then one can conclude that 20% of the patients respond to H5 peptides. Control samples (n=271) were obtained from two individuals in Vietnam and the United Kingdom who are not believed to have been exposed to H5N1 virus. None of these had H5N1-specific T-cell responses.
Curiously, only four subjects had both antibody and T-cell responses to H5N1 virus. The timing of sample acquisition with respect to infection is likely to be important for detecting responses. For example, antibody to H5N1 virus may not be detected earlier than 3 weeks after onset of disease, and T-cell responses may wane with time. It is also possible that abortive H5n1 infections in humans may lead to production of T-cells but not antibodies, as is seen in some individuals infected with HIV-1.
These findings provide additional evidence for subclinical human infection with influenza H5N1 virus. Exactly how many of people in the Vietnamese cohort were infected cannot be determined. Some of the H5-specific responses likely arose from previous H5 infection, while others may represent cross-reactivity with epitopes shared among H1, H3, and H5 viruses. The study also raises the interesting question of whether T-cell assays can be used as diagnostic tests for viral infections.
Powell, T., Fox, A., Peng, Y., Quynh Mai, L., Lien, V., Hang, N., Wang, L., Lee, L., Simmons, C., McMichael, A., Farrar, J., Askonas, B., Duong, T., Thai, P., Thu Yen, N., Rowland-Jones, S., Hien, N., Horby, P., & Dong, T. (2011). Identification of H5N1-Specific T-Cell Responses in a High-risk Cohort in Vietnam Indicates the Existence of Potential Asymptomatic Infections Journal of Infectious Diseases, 205 (1), 20-27 DOI: 10.1093/infdis/jir689

Pingback: [Crof's H5N1] Racaniello: More evidence for mild influenza H5N1 infections | Influenza Virus Mashup