
One of the first human studies on the effectiveness of Tamiflu was published about ten years ago. The human subjects (117 healthy adult volunteers, 18-40 years of age, with hemagglutination-inhibition antibody titers 1:8 or lower) were infected intranasally with a seasonal H1N1 strain of influenza virus. Some subjects were given Tamiflu or placebo 26 hours before infection, while others were given the treatment 28 hours after inoculation. Frequency of infection, viral shedding in nasal washes, and clinical symptoms (such as temperature, nasal stuffiness, runny nose, sore throat, sneezing, cough, breathing difficulty, muscle aches, fatigue, headache, earache/pressure, feverishness, hoarseness, chest discomfort, overall discomfort) were then determined.
Administration of Tamiflu 28 hours after inoculation reduced viral shedding and clinical symptoms, as shown in the following two graphs. Note that virus titers peaked in treatment and placebo groups at roughly 36 hours after inoculation. Viral titers declined more rapidly in individuals given Tamiflu, and no virus was detected by 60 hours after infection.
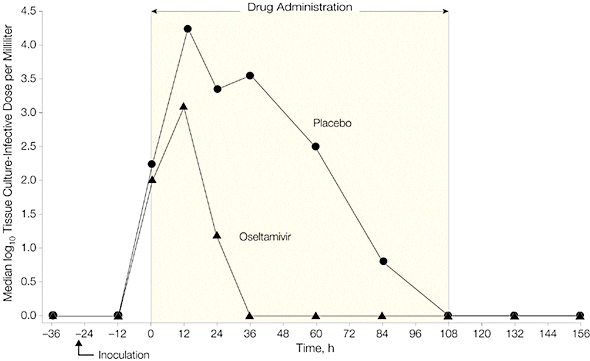

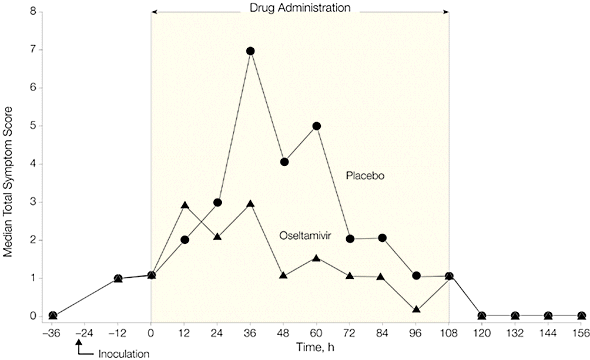

When Tamiflu was given before infection (prophylaxis), 8 of 21 individuals became infected (38%), while 8 of 12 individuals given placebo were infected (67%). None of the patients given Tamiflu shed virus in nasal secretions, or reported respiratory illness. Virus was shed by 50% of individuals in the placebo group, and a third had clinical symptoms of infection.
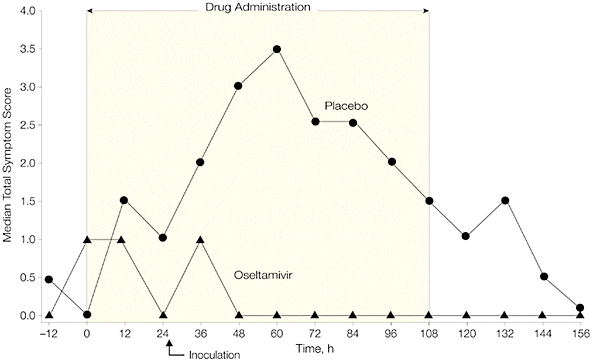

The authors concluded that “In these trials, prophylaxis and early treatment with oral oseltamivir were both associated with significant antiviral and clinical effects in experimental human influenza.” In other words, if you know you have influenza, Tamiflu works reasonably well.
The results of these studies justified the larger scale clinical trials which were subsequently done to provide the data needed for approval of Tamiflu in humans.
Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, Kinnersley N, Mills RG, Ward P, & Straus SE (1999). Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA : the journal of the American Medical Association, 282 (13), 1240-6 PMID: 10517426

Pingback: Tweets that mention Influenza neuraminidase inhibitors work -- Topsy.com
Pingback: uberVU - social comments
but the dayly routine is different. Most people start sneezing and go to a medic soon after. In almost all of these cases, it's way past 36 hours after inoculation. Most often they visit a medic about 1 or 2 days since onset of disease. So, in most of these cases, the clinical relevance of tamiflu is minimal. (you might better save it for hospital use, or early home-treatment/profylaxis for the immunocompromised)
i love the podcast by the way! follow it every week for over half a year now! thanks
Ewout Fanoy, MD infectious disease control, MHS, the Netherlands
Everyone here in France seems to be given oseltamivir, however long it takes them to go to their physician. I've seen lots of people be given oseltamivir literally days after onset. It seems to be the norm here and I'm somewhat baffled.
It's sometimes hard for people like me, who have a strong interest in science but are not actual scientists, to assess the authority of a given piece of litterature. It is a bit disheartening. Hopefully there are follow-ups.
Merry Christmasï¼
Everyone here in France seems to be given oseltamivir, however long it takes them to go to their physician. I've seen lots of people be given oseltamivir literally days after onset. It seems to be the norm here and I'm somewhat baffled.
It's sometimes hard for people like me, who have a strong interest in science but are not actual scientists, to assess the authority of a given piece of litterature. It is a bit disheartening. Hopefully there are follow-ups.
Merry Christmasï¼