If you have been following the results of my experiments on inhibition of rhinovirus replication by ZnCl2, you know that I’ve been trying to determine why concentrations of the salt higher than 0.1 mM are toxic to HeLa cells. I have found that 0.1 mM ZnCl2 does inhibit rhinovirus plaque formation but not sufficiently to be able to select resistant mutants. In today’s set of experiments I asked whether the presence of MgCl2 in the agar overlay potentiates zinc toxicity.
We always include MgCl2 (40 mM) in the agar overlay when assaying rhinoviruses, because it significantly improves plaque size. The following monolayers of HeLa cells were inoculated with 200 plaque-forming units of rhinovirus type 1a, then incubated at 32°C for 5 days. The effect of MgCl2 is remarkable.
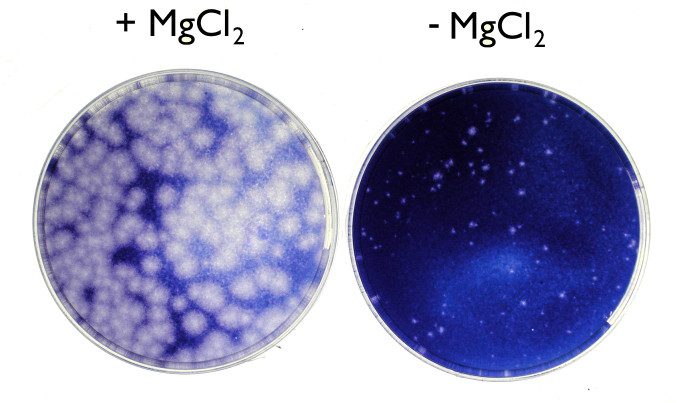

The use of MgCl2 to improve plaque formation of certain picornaviruses dates to the 1962 observation that the salt increases susceptibility of cells to poliovirus infection. It was later shown to enhance plaque formation of rhinoviruses.
Unfortunately, omission of MgCl2 from the agar overlay has no effect on zinc toxicity. As shown below, cell viability was still poor in the presence of 0.2 mM ZnCl2.

What’s next? One reader suggested that I try selecting HeLa cells in successively higher concentrations of ZnCl2 to obtain cells resistant to the toxic effects of the salt. This approach is under way. I will also attempt to propagate rhinovirus in cells covered with liquid growth medium rather than under agar. If 0.1 mM ZnCl2 is included in the culture medium, a good fraction of the viruses produced might be resistant to the salt. The virus produced in these infected cells will be used to infect fresh cells, also in culture medium with ZnCl2. After 5-10 passages in this manner the majority of the viral population should be resistant to ZnCl2. This is a more time consuming approach that the plaque assay, but might yield zinc resistant rhinovirus mutants.
Wallis, C., & Melnick, J. (1962). Magnesium chloride enhancement of cell susceptibility to poliovirus. Virology, 16 (2), 122-132 DOI: 10.1016/0042-6822(62)90287-8
Fiala M, & Kenny GE (1966). Enhancement of rhinovirus plaque formation in human heteroploid cell cultures by magnesium and calcium. Journal of bacteriology, 92 (6), 1710-5 PMID: 4289358

Very interesting, it seems that Mg is enhancing plaque size by displacing Zn, and by increasing zinc concentration the equilibrium flips back reducing the plaques again. I would think that the effect is due to a cellular metalloprotease important for viral life cycle, but a minimum activity of this metalloprotease is necesary for cell survival as well. Under this conditions an extracellular quelating agent will flip the plaques back to small size in the presence of Zinc, indicating that the metalloprotease is membrane attached or extracellular, then you can use KO cell lines or specific inhibitors. If it is not extracellular (most likely due to the virus life cycle), then you will see accumulation of an unprocessed viral protein that might be spotted in a 2D gel (compare with and without Zg).
Actually, I just found this:
1. J Virol. 1976 Apr;18(1):298-306.
Inhibition by zinc of rhinovirus protein cleavage: interaction of zinc with
capsid polypeptides.
Korant BD, Butterworth BE.
Zinic ions rapidly inhibit virus production in HeLa cells infected with human
rhinovirus type 1A and lead to the accumulation of human rhinovirus type 1A
precursor polypeptides. The degree to which cleavage of these precursors is
inhibited is directly dependent on the quantity of cell-associated zinc.
Proteolysis resumes after the removal of zinc-containing medium, and the
accumulated viral precursors are cleaved predominantly to stable virus
polypeptides. The precursors stabilized at the lowest zinc levels are those that
contain capsid protein sequences. Furthermore, added zinc is bound to human
rhinovirus type 1A capsids and prevents them from forming crystals.
Zinc-resistant mutants display antigenic alterations in coat proteins. These
results suggest that zinc complexes with rhinovirus coat proteins and alters them
so that they cannot function as substrates for proteases or as reactants in the
assembly of the virus particles.
Yes, I've seen that and similar papers. No genetic analysis was done
to prove the target of Zn inhibition, which is why I'm doing the
current experiments. In 1976 one could only make rather crude
conclusions about the target of Zn. For example, they say they have Zn
resistant mutants, but did not identify the mutation nor did they
provide little characterization. The fact that one Zn resistant mutant
no longer reacts with polyclonal anti-HRV sera tells me it was a
contaminant, probably poliovirus, which they also work with in that
paper.
Don't you love those 'old' virology papers in J. Bact!
When virology was simpler! I'm happy that they are online, because
they are gone from our library.
ProfVRR, you might want to check this one out re: research into zinc's effects on human and murine cells:
http://scienceblogs.com/neurotopia/2009/12/zica…
Yes, I've seen that and similar papers. No genetic analysis was done
to prove the target of Zn inhibition, which is why I'm doing the
current experiments. In 1976 one could only make rather crude
conclusions about the target of Zn. For example, they say they have Zn
resistant mutants, but did not identify the mutation nor did they
provide little characterization. The fact that one Zn resistant mutant
no longer reacts with polyclonal anti-HRV sera tells me it was a
contaminant, probably poliovirus, which they also work with in that
paper.
Don't you love those 'old' virology papers in J. Bact!
When virology was simpler! I'm happy that they are online, because
they are gone from our library.
ProfVRR, you might want to check this one out re: research into zinc's effects on human and murine cells:
http://scienceblogs.com/neurotopia/2009/12/zica…
ffffggggggggggg