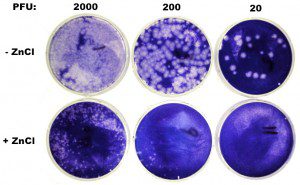

In the last experiment I confirmed the finding that 0.1 mM ZnCl2 inhibits plaque formation by rhinovirus type 1A. Based on the results of that plaque assay, shown in the figure at left, I’ve decided that this concentration of zinc isn’t sufficient to completely inhibit viral replication. Although 0.1 mM ZnCl2 blocked plaque formation when 20 or 200 pfu were inoculated on cells, many plaques arose on plates inoculated with 2000 pfu. These cannot be viral mutants resistant to zinc – there are too many of them. If there are 2000 plaques on the untreated plate, and 200 on the plate with zinc, that would mean that resistance to zinc arises at a frequence of 200/2000 = 0.1 or one in ten viruses. I would expect a mutation rate for an RNA virus to be more in the range of 1/1000 – 1/10,000.
I decided to repeat the plaque assay with higher levels of zinc in the agar overlay – 0.2, 0.3, and 0.4 mM. The results of that experiment are shown below.
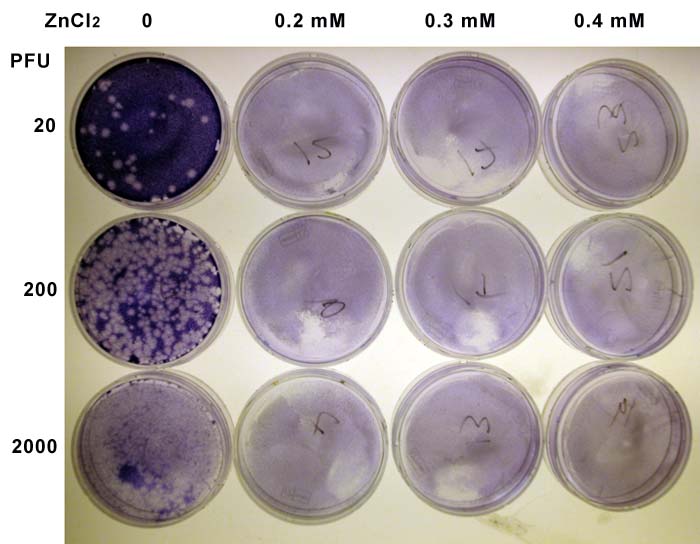

Unfortunately, the cells did not like the higher concentrations of ZnCl2! All the plates with zinc had very lightly staining monolayers – compare with the original experiment shown above – and no plaques were visible.
I was surprised that slight increases in the concentration of ZnCl2 would have such a dramatic effect on cell viability. The cells I used are HeLa cells, which are quite sturdy. Two other variables were changed in this experiment. I made a new stock of ZnCl2 – I took the 1 M stock I made for the first experiment and diluted it to 0.1 M. I doubt this made any difference. Second, the cell monolayers were less confluent than in the first experiment – about 70% of the surface of the cell culture dish was covered with cells. In the previous experiment, the monolayers were 100% confluent.
I repeated the experiment with HeLa cell monolayers that are 100% confluent. The results will be posted here in a few days.

Pingback: uberVU - social comments
Does anything happen if the virus is held in the ZnCl2 before infection? ie stocks of viruses diluted out of ZnCl2 before infection.
I haven't tried mixing virus with ZnCl2 before plating, but it's worth
a try. I'll do it next week.
How about first determining maximum Zn concentration that cells can withstand, then using that concentration for your plaque assay?
The ZnCl2 results seem consistent with other potential metal-ion based therapeutics, e.g. cobalt(III) hexammine, which also has an IC50 in the < 0.1 mM region against several types of viruses. Maybe that concentration level is something common to broad-spectrum metal-ion antivirals. You might be interested in running some tests in parallel.
That's what I'll be doing today – trying a range of ZnCl2 concentrations.
I've been following this Rhinovirus experiment you're performing. Please excuse my lack of knowledge: I thought HeLa cells are derived from a cervical cancer cell line. Rhinovirus causes common cold so I'm assuming it infects cells in the airway? What's the rationale of using HeLa cells? (I also noticed the original publication used HeLa cells as well.)
It's a perfectly reasonable question. Clearly HeLa cells, being of
cervical origin, are not relevant to the biology of rhinovirus. They
are very convenient for studying aspects of viral replication and
genetics that depend upon efficient viral replication. In the case of
these experiments, it is easy to plaque rhinoviruses in HeLa cells and
to select for drug resistant mutants. But your comment will provoke me
to pull some respiratory cells out of the freezer and see if I can get
rhinovirus to form plaques on them.
In counting the PFU and assaying for viral titre, HeLa cells sounds like a great choice. However, when the goal is to try to identify biologically relevant mutants to find out zinc's mechanism of action, I am prone to think respiratory cells would be a better choice? Again, I don't know much about cell cultures and so there may be factors that I have not considered (for instance, how much will a virus be affected when different cell lines are used). Perhaps you can test the growth of some respiratory epithelial cells under a range of ZnCl2 concentrations and see whether this model is viable… Good luck! I'll keep reading! By the way, I love your blog, TwiV and TwiP. I'm a PhD student in HIV. 🙂
You make very good points, and I do plan to look at the effects of
ZnCl2 in a respiratory cell line. If the sole target of ZnCl2 is a
viral protein or RNA, then it might not matter what cell types the
study is done in. For this reason I decided to get the project started
in a very easy cell line to work with, HeLa cells, and then compare
the findings in a respiratory line. Thanks for reading and listening,
and good luck with HIV.
I've been following this Rhinovirus experiment you're performing. Please excuse my lack of knowledge: I thought HeLa cells are derived from a cervical cancer cell line. Rhinovirus causes common cold so I'm assuming it infects cells in the airway? What's the rationale of using HeLa cells? (I also noticed the original publication used HeLa cells as well.)
It's a perfectly reasonable question. Clearly HeLa cells, being of
cervical origin, are not relevant to the biology of rhinovirus. They
are very convenient for studying aspects of viral replication and
genetics that depend upon efficient viral replication. In the case of
these experiments, it is easy to plaque rhinoviruses in HeLa cells and
to select for drug resistant mutants. But your comment will provoke me
to pull some respiratory cells out of the freezer and see if I can get
rhinovirus to form plaques on them.
In counting the PFU and assaying for viral titre, HeLa cells sounds like a great choice. However, when the goal is to try to identify biologically relevant mutants to find out zinc's mechanism of action, I am prone to think respiratory cells would be a better choice? Again, I don't know much about cell cultures and so there may be factors that I have not considered (for instance, how much will a virus be affected when different cell lines are used). Perhaps you can test the growth of some respiratory epithelial cells under a range of ZnCl2 concentrations and see whether this model is viable… Good luck! I'll keep reading! By the way, I love your blog, TwiV and TwiP. I'm a PhD student in HIV. 🙂
You make very good points, and I do plan to look at the effects of
ZnCl2 in a respiratory cell line. If the sole target of ZnCl2 is a
viral protein or RNA, then it might not matter what cell types the
study is done in. For this reason I decided to get the project started
in a very easy cell line to work with, HeLa cells, and then compare
the findings in a respiratory line. Thanks for reading and listening,
and good luck with HIV.
hi
I intend to do plaque assay for adenovirus carrying specific target gene, can anybody suggest any negative control, in addition to vector without gene?
nice theme. but it takes a while to load
Pingback: binary options trading
Pingback: Factor auto notes