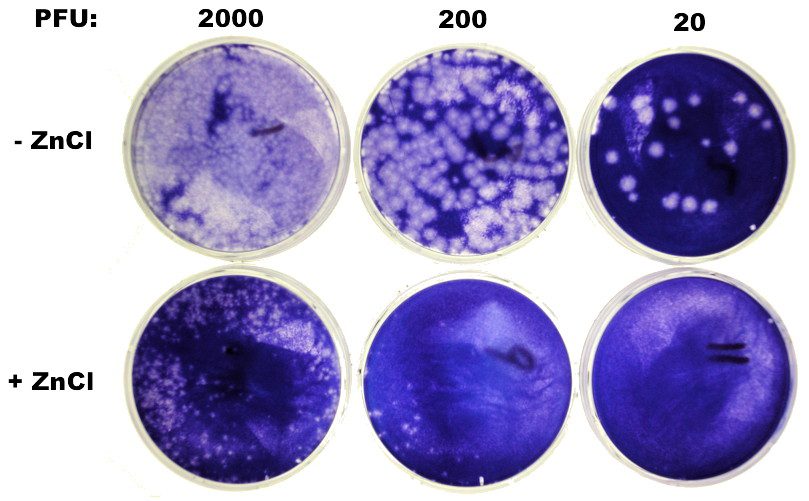

I am interested in understanding how zinc inhibits rhinovirus replication. Answering this question could lead to new ways to prevent common colds caused by these viruses. The first step was to reproduce the effect of zinc in my laboratory with my stocks of rhinovirus. I selected rhinovirus type 1a for my initial experiments because we’ve worked with this serotype in the past: we know the genome sequence and how the virus behaves in a mouse model. I started by doing a plaque assay with and without zinc in the medium. I prepared tenfold dilutions of virus and inoculated separate monolayers of HeLa cells with 2000, 200, and 20 plaque forming units. After allowing the virus to attach to cells for 45 minutes, I added an agar overlay to the cells with or without zinc chloride (ZnCl2). I selected 0.1 millimolar ZnCl2 because that is the concentration which had been reported to effectively inhibit plaque formation by rhinovirus type 1a. The plates were incubated for four days at 32°C and then stained. The results are shown in the photo. Plaque assays are typically done in duplicate but for simplicity only one plate of each dilution is shown.
Twenty plaques were observed on the highest dilution of virus plated in the absence of ZnCl2. Ten-fold lower dilutions produced increases in plaque number, although the plaques are too numerous to count. In the presence of ZnCl2, no plaques were observed on cells inoculated with 20 PFU. A few plaques are observed on the intermediate dilution and many more on the lowest dilution. Plaques observed in the presence of ZnCl2 are smaller than those observed in the absence of the metal.
What do you think is going on here, and what should I do next? If you’ve kept up with virology 101 you have all the tools to answer these questions. Please post your thoughts in the comments section.
KORANT, B., KAUER, J., & BUTTERWORTH, B. (1974). Zinc ions inhibit replication of rhinoviruses Nature, 248 (5449), 588-590 DOI: 10.1038/248588a0

Repeat the same experiment using different kinds of virus and see whether you'll observe a similar effect? (distinguish between rhinovirus-specific factor vs. host factor)
Pingback: uberVU - social comments
Insert zinc-plated nasal stents and walk amongst crowds with impunity?
Pingback: Tweets that mention Zinc inhibits rhinovirus replication -- Topsy.com
Isolate virus from the plaques, grow it up and sequence.
Pretreat the cells with ZnCl2 in order to mimic a prophylactic treatment.
Perform a cinetic of adding ZnCl2 “x” time after infection.
Perform a dose ranging in order to optimise ZnCl2 antiviral effect and cell toxicity.
Identify which step of virus life cycle is affected by ZnCl2.
Thanks to having performed this experiment and share results with us.
I'm just tickled to hear that you're back in the lab doing experiments. Congratulations!
Oh, and of course I'd do pretty much what others have already suggested here: a time-course of infection, adding the zinc at different points to see when the block occurs, plus isolation of zinc-resistant mutants. After the initial time-course results, it might also be interesting to see if you can reverse the block by chelating the zinc, which would reveal whether zinc inhibition really inactivates the virus or merely “pauses” its cycle. I might also see if other metals inhibit the virus's growth.
Very interesting. I imagine that as a researcher your first goal is to find out how the zinc works, but I do have a question that I hope you don't mind my asking .
What about the June 2009 FDA's report that Zicam's zinc nasal spray has been implicated in people losing their sense of smell? Do you already know how this side effect can be circumvented and avoided?
I don't want to use zinc to inhibit rhinoviruses. The goal is to find how how zinc works, then design molecules that mimic what zinc does without the side effects.
What's a “cinetic”?
Just a WAG but I would look for protease inhibition by Zn++. Both 2A and 3C are cysteine proteases (iirc) so they would be my first guess as the target for Zn++ inhibition of rhinovirus replication. But, I would be surprised if that had not already been done!
sorry for this french neologism, it was kinetic !
“Kinetic” means 'pertaining to motion'. In this case, it refers to adding zinc at different times after infection.
At the molecular level it would be quite interesting to probe the mechanism of action and where zinc ions are going and binding. These days we have nice tools to examine the speciation of transition metals in their interactions with biological systems. For instance, we could start by finding out the group of proteins or receptors that Zn is binding to by using an ICP-MS system as a detector (interfaced) with your HPLC protein separation system. This will tell us where Zn is in the chromatographic profile and we will follow up with LC/MS-MS or LC/Q-TOF analysis to identify the proteins. For Zinc the detection limit of an ICP-MS system is less than 1ppt; probably low enough for mechanist studies. I would love to try it!
At the molecular level it would be quite interesting to probe the mechanism of action and where zinc ions are going and binding. These days we have nice tools to examine the speciation of transition metals in their interactions with biological systems. For instance, we could start by finding out the group of proteins or receptors that Zn is binding to by using an ICP-MS system as a detector (interfaced) with your HPLC protein separation system. This will tell us where Zn is in the chromatographic profile and we will follow up with LC/MS-MS or LC/Q-TOF analysis to identify the proteins. For Zinc the detection limit of an ICP-MS system is less than 1ppt; probably low enough for mechanist studies. I would love to try it!
Pingback: Zinc and rhinovirus replication
Pingback: Rhinovirus and zinc part 5: Magnesium is not the culprit
Zinc is needed for the metabolism of nucleic acids and the synthesis of proteins. It is an integral part of the human DNA for cell division and synthesis. Zinc is anti-bacterial, anti-viral and is found in all the body fluids, including the moisture in the eyes, lungs, nose, urine, and saliva. liquid zinc
Zinc Chloride is widely used as application in textile processing, chemical synthesis and metallurgical fluxes.
Pingback: Ugh. - Breast Augmentation, Breast Implants, and Plastic Surgeons Forums
I’m seeing that only more than 4 years after it was done (and only ’cause I have a freaking cold now), but… You did check that it is not the Chloride that makes all the work, right? 🙂
A voice from the distant past: James C. Kauer, chemist…..Around 1973, approximately, I handed a sample of a commercial organic compound, maleopimaric acid, used in the papermaking industry (mixed with its zinc salt we learned later) to my friend and coworker Bruce Korant, a virologist, as a a possible inhibitor of an enzyme involved in rhinovirus replication which he had discovered. (The idea of using the maleopimaric acid came from a report that this material inhibited certain,perhaps similar enzymes involved in the complement cascade.) Dr. Korant soon discovered that the material indeed worked. When I purified the submitted material we found that it was only the zinc salt, and not the maleopimaric acid which had the activity. Every zinc salt which was tested had the activity! The results were published in NATURE in 1974. The work was soon followed up by additional publications by Dr. Korant and his collaborator, Byron Butterworth in the virology literature.