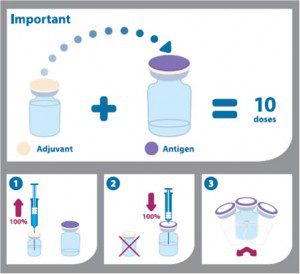

The Canadian vaccine, called Arepanrix H1N1, is supplied in two parts. One contains inactivated H1N1 influenza virus, and the second consists of AS03 adjuvant (DL-a-tocopherol, squalene, polysorbate 80). Before injection the virus and adjuvant are mixed. The vaccine is provided in 10-dose vials and therefore contains thimerosal. More information on the amounts of these components can be found at the Canada Health website (“Product Information Leaflet Arepanrix„¢ H1N1 AS03-Adjuvanted H1N1 Pandemic Influenza Vaccine”).
Health Canada approved the vaccine based on limited clinical testing, under the provision of an interim order. It is expected that additional safety data will be provided at a later date. The interim order that allows use of Arepanrix H1N1 is based on safety and immunogenicity data on an H5N1 vaccine prepared with AS03 adjuvant in adults and in children, and on 2 separate studies of an H1N1 vaccine prepared with AS03 adjuvant in adults. Canada Health assumes that Arepanrix H1N1 with adjuvant will behave similarly in children and adults as these two tested vaccines, and hence issued the interim order of approval. Let’s examine some of this information.
Adverse reactions: Two studies on H1N1 vaccine were conducted in adults 18-60 years of age. Adverse effects reported included inoculation site pain, redness, swelling, fatigue, headache, arthralgia, myalgia, shivering, and sweating. In general the reactions were reported more frequently in those receiving vaccine with adjuvant. For example, inoculation site pain was reported in 88.9% of 63 individuals who received vaccine with adjuvant, and in 59.1% of 66 who received vaccine without adjuvant. Similar observations were obtained in the second study with 124 individuals.
More extensive studies compared adverse effects of inoculating H5N1 vaccine plus adjuvant in 3,500 adults; the control group was inoculated with buffer. A similar range of symptoms was reported, the most common pain (73%) and muscle aches (33%). Incidence was less in those who received placebo (pain 12%; muscle aches 11.8%). In a separate study, ~300 children 3-5 and 6-9 years of age were given a full or half dose of H5N1 vaccine plus adjuvant. Pain, redness, swelling, fever, drowsiness, irritability, loss of appetite and shivering were more common in those who received the full dose of vaccine. No serious adverse effects caused by the vaccine were recorded.
Immunogenicity: In two separate studies in adults, the H1N1 vaccine lead to seroconversion of 97 and 98.4% of test subjects with or without adjuvant (conversion defined as an HI titer greater than or equal to 1:40). No immunogenicity studies of the H1N1 vaccine in children have been reported. There have been studies in children inoculated with an H5N1 vaccine, but I won’t consider those because the clinical experience is quite different from H1N1 vaccines. The full report can be found here.
Summary: In adults, the H1N1 vaccine approved in Canada induces protective immune responses. The immunogenicity of the H1N1 vaccine in children remains to be determined. In both adults and children, the use of adjuvant leads to more frequent adverse reactions but these are not serious. Consequently Canada Health recommends that children 3-9 years old should be given two half doses of vaccine three weeks apart, and children 10-17 years should receive the full dose (0.5 mL).
Canada has purchased 1.8 million doses of inactivated H1N1 vaccine without adjuvant, but those will not be available until November.

Hats off to you for your accurate information and excellent answers! I have a 17 month old and a 4 year old who at this point have not received the (CANADIAN) vaccine, as I have concerns over the lack of scienctific research in this age group and the risks of squalene mainly. My children get vaccinated against the diseases that vaccines prove to be effective for(i.e. all except chicken pox and flu). My question is many MDs are saying that if your child has something right now , there is a 90% chance it is swine flu. I was in the ER 2x last week with baby with croup and then fever 2 days later. I did not the administer the Tamiflu that was prescribed and she is back to normal. What are your thoughts on the possibility of that infection having been swine flu?
Hi there, my physician says the adjuvanted vaccine in Canada provides cross-immunity to mutations of H1N1 virus but not the the unadjuvanted one. I'm trying to decide which one to give to my 16 month old son. He also said the immunity won't be as good with the unadjuvanted one even after two doses! Does this mean many kids in his age group are not well protected in the states because there is no adjuvanted vaccine there? Is the Canadian population better protected against mutations of this virus? Thank you.
I'm not sure that 90% is accurate. According to Google flu trends
(http://www.google.org/flutrends/us/) flu-like illness has already
peaked and is declining. Which is not to say it won't peak again, but
I think at the moment it's not correct to assume that 90% of
respiratory infections are influenza.
Both vaccines, with or without adjuvant, provide good protection. The
vaccine without adjuvant contains more viral antigen and therefore
it's not correct to say that immunity induced by this vaccine won't be
as good as that induced by the vaccine with adjuvant. The adjuvant was
included in the Canadian vaccine so that more doses could be produced.
Protection of children in the US and Canada will not differ because of
the different immunization regimens.
Thank you. Is it true that you might get cross immunity from the adjuvanted one? How does this happen if they both have the same antigen? In Canada they now recommend not using the unadjuvanted one for his age group because immunity is not as good! We're concerned the adjuvant might cause an autoimmune disease etc. in the future. Any long term studies on this for his age group?
Pingback: GlaxoSmithKline influenza H1N1 vaccine approved
I took my 18 month old daughter for the half dose of the adjuvanted vaccine almost 21 days ago. It was a very difficult decision for us to make as we were very concerned that no studies in this age group had been completed. She had no adverse reactions but we are not sure that we want to give her the other half. What immunity will she have and does this one half dose help to prevent H1N1 from being more serious if she were to catch it?
My daughter is 9 mos old and she did not have a reation to the shot. However, she developed more excema on her arms and chest days after..I didn't think it had anything to do with the vaccine until I saw your post here.
Novartis has said that their half dose (3.75 micrograms) is enough for
protection. At worst reinfection might occur but the symptoms would be
less severe. Balance your concerns with that information.
My daughter had the shot about a month ago, the excema got worse a few days later and eventually covered about half of her body. Its finally going away now. Doctor says its unrelated but it really makes me wonder! Her diet has not changed at all over that time period!
I have a 1, 3 and 5 year old who have all had the 1/2 dose of the Canadian adjuvanted vaccine. Canada is now advising that children ages 3 to 9 years old only need the one 1/2 dose and children between the ages of 6 months and under 3 years should have the 2nd 1/2 dose. I'm confused how this makes any sense and want to better understand the logic behind this change in dosing. Shouldn't the older children who weigh more have a greater need for the 2nd dose and aren't they at a greater risk for contracting H1N1 since they are school aged?
This fellow Canuck would like to know as well. It would be nice to only have to vax the babies once. Any advice out there?
On November 13 Health Canada updated their H1N1 vaccine dosing
recommendations. Previousl children from 6 months to 9 years were
recommended to receive two half doses. Now only those 6 months-3 years
of age need both doses; from 3 years to 9 years only one half dose.
This recommendation is based on the results of clinical trials which
indicate that in the 3-9 year age group a single dose is protective.
Health Canada also notes: “These recommendations may be updated as
more information becomes available.” Weight is not the deciding
factor; it's how the children respond to the vaccine. Older children
(3-9 years) may have a stronger response than younger children because
they've had flu before.
Twice for babies according to Health Canada. But in the four weeks
until the next dose the recommendations might change.
Thanks for the clarification.
On November 13 Health Canada updated their H1N1 vaccine dosing
recommendations. Previousl children from 6 months to 9 years were
recommended to receive two half doses. Now only those 6 months-3 years
of age need both doses; from 3 years to 9 years only one half dose.
This recommendation is based on the results of clinical trials which
indicate that in the 3-9 year age group a single dose is protective.
Health Canada also notes: “These recommendations may be updated as
more information becomes available.” Weight is not the deciding
factor; it's how the children respond to the vaccine. Older children
(3-9 years) may have a stronger response than younger children because
they've had flu before.
Twice for babies according to Health Canada. But in the four weeks
until the next dose the recommendations might change.
Thanks for the clarification.