The Advisory Committee on Immunization Practices (ACIP) has released its recommendations on the use of influenza A (H1N1) 2009 monovalent vaccine. Here is my summary of the salient points.
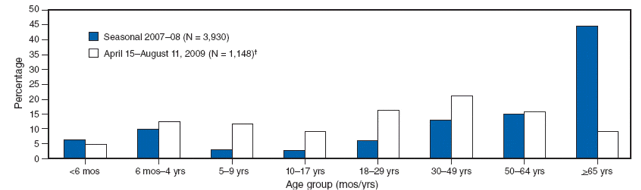
The report begins with information on who is contracting pandemic 2009 H1N1 influenza. The median age of persons with laboratory-confirmed infections in the United States is 12 years. The highest incidence of infection is among persons aged 5-24 years, and the lowest is in persons over 65 years of age. Similar findings have been reported in other countries. Comparison of the age distribution of hospitalized persons with laboratory-confirmed novel influenza A (H1N1) also shows a striking difference compared with seasonal influenza, as shown in the figure.
As we have discussed previously, the reduced susceptibility of older individuals to infection with the 2009 pandemic virus is likely a consequence of their previous exposure to an antigenically cross-reactive strain of the virus.
Another important point is that the seasonal influenza vaccines will not provide protection against the 2009 pandemic influenza H1N1 virus. This trivalent vaccine (containing seasonal H1N1, H3N2, and influenza B virus strains) is currently being distributed. Why would anyone consider receiving this vaccine? Seasonal influenza A H1N1 and H3N2 viruses continue to circulate in the US at low levels (as shown here), and it’s not yet clear whether they will disappear in the fall. Consequently the committee believes that all individuals recommended for seasonal influenza vaccine, including those 65 years of age and older, should receive the seasonal preparation as soon as it is available.
The report suggests that two doses of the vaccine against the 2009 pandemic H1N1 strain, spaced 21 days apart, will be required for full protection of children and young adults. Two doses of seasonal influenza vaccines are typically required to induce immunity in unvaccinated persons less than 9 years of age, because young children have not been infected with the virus and are not immunologically primed. The committee speculates that vaccines containing an adjuvant probably will not be used initially, and if they are needed, an Emergency Use Authorization will be required.
Who should receive the vaccine against the pandemic H1N1 strain? Since availability will be limited, the committee recommends that individuals in the following five groups should be first:
- Pregnant women,
- Persons who live with or provide care for infants <6 months old
- Health-care and emergency medical services personnel
- Persons aged 6 months – 24 years
- Persons aged 25 – 64 years who have medical conditions that put them at higher risk for influenza-related complications
These five target groups make up about 159 million persons in the US. Because sufficient vaccine will not be available to immunize this many people, a subset of them should be given priority:
- Pregnant women
- Persons who live with or provide care for infants <6 months old
- Health-care and emergency medical services personnel who have direct contact with patients or infectious material
- Children aged 6 months – 4 years
- Children and adolescents 5 – 18 years old who have medical conditions that put them at higher risk for influenza-related complications
These groups comprise about 42 million persons.
The committee does not recommend simultaneous administration of infectious, attenuated vaccines against seasonal and novel influenza A (H1N1) virus – probably to avoid the emergence of reassortants between the different viruses. However, you may receive both inactivated vaccines against seasonal and pandemic influenza viruses – one in each arm.

hi
I'm clinical biochemist and glad to find here
I want inform my blog readers about A flu pandemi and need to read more about it and be well-informed.
I think here is good place for that
hummm… my blog is in persian
Vincent,
Considering that 2009-H1N1 has just about completely displaced seasonal H1N1 and there are considerable concerns of a H3N2 mismatch (see link below) – it is likely that the only protection that the seasonal vaccine may confer is for influenza B. Why bother? The best I can come up with is because influenza is very unpredictable??
C:Documents and SettingsdpattieDesktopH3N2A Novel H3N2 Influenza A Variant Emerges (07-31-2009) – Center for Biosecurity of UPMC.mht
Here is the correct link:
http://www.upmc-cbn.org/report_archive/2009/07_…
I want to check something out. A nurse who is giving seasonal influenza shots this month told me privately that the seasonal vaccine is only effective for 3 months, which is why they give it out in October usually. That would mean that you could still get a seasonal flu strain in February and March. What is the number of months the seasonal flu vaccine is effective? Thanks.
http://www.newfluwiki2.com/diary/1684/
http://www.ncbi.nlm.nih.gov/pubmed/18275271?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum%5Dhere
http://www.medscape.com/content/2002/00/44/44/444417/444417_fig.html
(figure 3)
See the excellent article in the PubMed citation from by gsgs below in
his email. The idea that vaccine induced immunity declines in ~4
months applies largely in the elderly population; but that appears to
be incorrect, based on the study.
See the excellent article in the PubMed citation from by gsgs below in
his email. The idea that vaccine induced immunity declines in ~4
months applies largely in the elderly population; but that appears to
be incorrect, based on the study.
Pingback: ACIP recommendations on monovalent H1N1 vaccine | H3N2FLU.US