
Delwart and his colleagues examined the purity of a number of human vaccines:
A little historical perspective about why we decided to analyze these vaccines even though there was absolutely no indications whatsoever that they are at all unsafe. Our intent was to use the latest technologies to show that live attenuated vaccine only contained the expected viral genomes and no other.
The Delwart laboratory obtained samples of eight infectious attenuated viral vaccines from the manufacturers: oral poliovirus vaccine (OPV, Bharat Biotech), rubella (Meruvax-II, Merck), measles (Attenuvax, Merck), yellow fever (YF-Vax, Sanofi Pasteur), human herpes 3 (Varivax, Merck), rotavirus (Rotarix, GlaxoSmithKline; and Rotateq, Merck) and multivalent measles-mumps-rubella (MMR-II, Merck). The vaccines were treated with DNAse and RNAse to remove nucleic acids that are not protected by viral capsids. Nucleic acid was then extracted from the vaccine, amplified by polymerase chain reaction, and subjected to DNA sequencing. A total of 501,753 sequence ‘reads€™ were done.
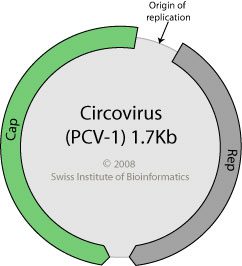
The sequence analysis revealed the expected vaccine strains in each preparation, and in three cases, other unexpected viral sequences. The retrovirus avian leukosis virus was found in the measles vaccine, but at a very low level (700 nucleotides from 4 sequence reads). A virus similar to simian retrovirus was identified in Rotateq (276 nucleotides from 1 sequence read). Significant levels of porcine cirovirus 1 were found in Rotarix. The entire viral genome sequence was deduced from 6344 sequence reads, comprising over 40% of the reads done for that vaccine.
The avian leukosis virus sequences found in the measles vaccine are in intact virions, as DNA treatment of the vaccines did not prevent their detection by polymerase chain reaction (PCR). However simian retrovirus DNA was not detected by PCR after DNAse treatment of Rotateq. Therefore the viral DNA detected in Rotateq vaccine most likely originates from host DNA present in the vaccine preparation. The cells used to produce Rotateq are Vero cells – African green monkey kidney cells. A defective form of simian retrovirus DNA, called proviral DNA, is integrated into Vero cell DNA.
How did a porcine virus contaminate Rotarix, which is produced in Vero cells? The answer is not known, but the authors speculate that the culprit might be porcine trypsin, which is used during the propagation of Vero cells. Over 100,000 porcine circovirus 1 DNA molecules were detected in each vaccine dose, fully 10 times higher than the amount of rotavirus present. However, it€™s not known if the porcine circovirus present is infectious.
There is little evidence that the presence of porcine circovirus 1 DNA in Rotarix constitutes a danger. Because both porcine circovirus 1 and 2 are common in healthy pigs, it€™s likely that humans are frequently exposed to these viruses by eating pork or when workers at swine farms inhale pig feces. Both viruses are often found in human stool. Whether or not these viruses can replicate in humans is currently a matter of debate.
According to the FDA, In four to six weeks, FDA will convene an expert advisory committee and make additional recommendations on the use of rotavirus vaccines. What do they mean by this statement? According to Delwart,
I believe that GSK and FDA are now testing whether PCV1 in Rotarix can replicate in human cells and/or determine if vaccinated children are sero-positive to PCV1. If those sorts of tests are negative it will indicate that PCV1 is most likely not infectious for humans and therefore not a health concern in Rotarix.
Delwart believes that his work should not cast a negative light on viral vaccines:
For now it is my hope that people will not interpret this study as putting vaccines in a bad light but rather as reflecting the intense attention placed on vaccine safety using all the latest tools. It is only because of cheaper sequencing that these sorts of studies are possible. People should not be alarmed or over-react to every virus detection unless there is concrete evidence of human infection and pathogenicity. Viral metagenomics can both discover new viruses and improve the safety of existing vaccines. Its use early in the development of vaccines should help ensure their purity.
Victoria, J., Wang, C., Jones, M., Jaing, C., McLoughlin, K., Gardner, S., & Delwart, E. (2010). Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. Journal of Virology DOI: 10.1128/JVI.02690-09

Comments are closed.