One of the most important procedures in virology is measuring the virus titer – the concentration of viruses in a sample. A widely used approach for determining the quantity of infectious virus is the plaque assay. This technique was first developed to calculate the titers of bacteriophage stocks. Renato Dulbecco modified this procedure in 1952 for use in animal virology, and it has since been used for reliable determination of the titers of many different viruses.
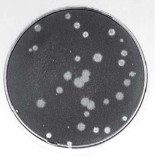

The titer of a virus stock can be calculated in plaque-forming units (PFU) per milliliter. To determine the virus titer, the plaques are counted. To minimize error, only plates containing between 10 and 100 plaques are counted, depending on the size of the cell culture plate that is used. Statistical principles dictate that when 100 plaques are counted, the sample titer will vary by plus or minus 10%. Each dilution is plated in duplicate to enhance accuracy. In the example shown below, there are 17 plaques on the plate made from the 10-6 dilution. The titer of the virus stock is therefore 1.7 x 108 PFU/ml.
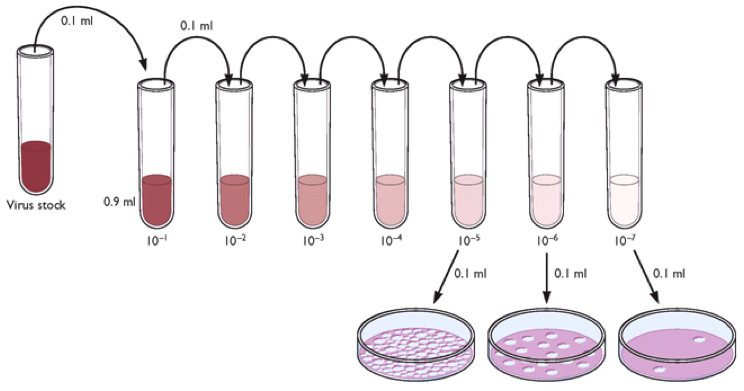

Next we’ll consider how the plaque assay can be used to prepare clonal virus stocks, a step that is essential for studying viral genetics.
Dulbecco, R., & Vogt, M. (1953). Some problems of animal virology as studied by the plaque technique. Cold Spring Harbor Symp. Quant. Biol., 18, 273-279

The number of plate form represent the number of virus OLAJIDE OLUROPO
If you can find some chikungunya-specific antiserum or monoclonal antibodies you could do an immunostaining procedure to detect foci of virus infection, instead of simply staining with crystal violet to see live vs. dead cells.Â
You can also try using an overlay of carboxymethylcellulose or Avicel (it’s a brand name for a mixture of cmc + insoluble cellulose) because they form suspensions that are viscous enough to stop the virus from moving around, but still liquid and can be aspirated. As opposed to solid agarose overlays which can be a bit tricky to peel off without messing up your cells.Â
Actually isn’t the value 1.7x10e-9?
i have one question if you can help me i shall be very thankfull to you for this act my question is
the
bacterial OD value is 0.4 which is equivalent to 1*10^8 cfu/ml and the
bacteriophage valuse is 1*10^9 and i want the MOI valuse 0.1 then how i
can calculate it i am waiting for your reply.
hi sir please explain the quantification,chaion virusracterization,and purificat
hi sir,i am bsc microbiology student. and my problem is that ,now in msc we have done the practical of isolating the phagefor about 10 times but still we failed and still could not isolate the phage .so i want standard procedure and guidance.thank u for this post.
w0w its so amazing !!!
Hi Sir,
Nice. Can you please also upload protocol for calculating viral titers after isolating from mouse organs (like Spleen or Lung)? I want to know about how to isolate viruses from organs and what solutions can I use for that? Thanks
hello sir,
I am testing chickungya virus titre by plaque assay method could you please tell me that how to minimize the variation between replicates and shall i consider 100 plaques for calculation or shall i consider in between 10-100 please help me in this aspect
You should count between 10-100 plaques (that is for a 10 cm plate).Â
شكرا علي الكلام العلمي الجميل ده جزاكم الله خير
بس ياجماعه Øد ممكن يجاوبني علي سؤال ده اذا سمØتوا اØنا عارÙين ان الÙيروس مش بيعيش الا داخل خلايا العائل طيب ازاي بيعيش علي ميديا عاديه اØنا اخدنا التجارب ٠المعمل بس الÙيروس مش بيعيش علي ميديا لكن بيعيش Ù عائل ياريت Øد يجاوبني
Rodrigo
To determinate the presence of virus, you should determinate the E2 and E6 genes. So, you can determinate if HPV is in integrated form or episomal status.
The load viral is determinated by the quantification of E6 copy number
how to calculate virus copy number?
Hi, i’m a master student,
My problem is that, at the end of my plaque i have some damage on the cells. Not all the bottom of the plate is intact, almost 10-30% of the bottom of my plate are damaged.
Please what are the possible causes of that?
Thank you
Pingback: Renato Dulbecco, 1914-2012
baterica yum
I was wondering what stops a plaque from continually getting bigger?
The agar overlay is sometimes too hot when you put it on the first one or two plates and this can kill the cells. Also, are the cells confluent when you perform the assay? Another thing it could be is drying out of the cells during the absorption period. Be sure to shake the plates every 15 min or so in order to disperse the inoculum over the entire well.
I’m trying to set up a focus forming assay. For the host cell the agarose seems to make them sick. Is the agarose strictly required or could I set up this test with out the overlay?
Hi sir, I would like to know I can do plaque assay for all type virus or not. if there are any perincipals about use of this method for different viruse , please tell me.
Thanks a lot for your help
Have a nice time
Hi sir.I am a graduated student in anhui medical university in China.thank you for your presentation.I wonder if you have any animation about virus neutralization tset.It is appreciate of you send this to my email:zwc2511109@163.com.
interms of a practical report results if the plaques are too many do you write too many to count?
Yes.
hi, i am looking for a simple practical for 16/17 year olds to perform to show the viral plaque counts on solid media.please help.
wat is this
can u stop this internet
ahhhhhhhh
how agar is laid on the palque please
virus can live on the cells that contain the positive receptor for that virus and the medium is a nourishment for the virus and the positive receptor cells
virus live on media containing cell and its name according to that cell infected it such as(colliphage staphyllococcous aureas phage and so on) every cell have spesific resepter
I would like to use the plaque assay for vaccinia virus. How do you know what percentage of agar to use? and secondly does anyone have a paper or protocol for plaque assays for poxviruses?
Pingback: The wall of polio
Hi sir,would you mind telling me what the factors determine the viral plaque size and i wonder the same virus but other serotypes can result in different plaque size or not,and why?
thx
Thank you for this protocol – very helpful. I am an instructor for a microbiology practical course and am planning on doing this plaque assay with the students. Problem is, we are only scheduled to meet once a week, on Friday mornings. If they incubate the plates Friday morning, and I come in and put them in the fridge on Saturday, will that keep the plaques stable until the following Friday? Many thanks for any advice you can offer!
Thank you very much Prof. Racaniello.
Is the range (10-100 plaques) for plaque assay of animal viruses only or even
bacteriophages? In my experiment with bacteriophages, several dilutions gave me
plaques between 10 and 100, so which dilution shall I use to calculate the
phage titer?
Hi Sir, I need a help. I have been done a plaque assay to detect bacteriophage in water sample by using double layer agar (DLA) method twice. I’ve used TSA and E.coli as a host. But there were no result obtained. I didn’t know what’s wrong with my experiment
Thanks in advance for your help. I do really appreciate it..
Øبيبي الميديا مو عادية
الميديا نضي٠عليها بكتيريا بالأول وبعدين نوضع الÙايرس عليها
Hello, someone could explain me how caluclate the detection limit of plaque assay according to the Poisson formula?
am a msc virology student and have an assignment to discuss methods to measure size of a virus.Any one with a link or answers would be very helpful@victormburu@gmail.com
Hi Vincent! Thanks for posting this explanation. I just did my first plaque assay today and was looking for more explanation into how it works and how to graph the results. I figured the Virology blog would have some answers and as usual I wasn’t let down. Thanks for putting all of this information together for us budding virologists.
Sir,
I had prepared virus lysate which was giving titre of around 10^12 previously. But now that is after around 3 months sometimes it suddenly boost up to 10^14 for the day or two and again gets back to normal. I can not figure out what is the reason behind it. Please help
is it favourable to use plaque assay to detect viruses in raw eaten fruits and vegetables???? help plz
Pingback: Now playing: Viral plaque formation
Pingback: The Wall of Polio, version 3.0
Hi Sir, good day! I’m a 4th year Biology student. My question is why should we use a hard agar with a soft agar overlay technique to demonstrate plaque formation?
The hard agar is the substrate for bacterial growth. The soft agar is used to mix the bacteria and phage dilutions which is then spread over the hard agar. The bacteria form a lawn with clear zones cause by viral growth. Note, this is the plaque assay for bacterial viruses. For animal viruses, only a single agar overlay is used.
Hi Sir, I need a help. I have been done a plaque assay to detect bacteriophage MS2 by using double layer agar (DLA) method . I’ve used E.coli as a host. 500 ul host add 5 ml LB medium as top agar. The plaque not very clear (hard to count but can vaguely see ) , but I had the same experiment before and also had good outcome. I suspect that the problem is virus infectivity. Can you give me some suggestions.
thank you
could someone guide me for Viral bank prparation of Bovine parainfluenza virus using BT cells? also the plaque assay
Fix infected cells (cold Ethanol+Acetone 30/70%) after several days, and do PLA-ELISA with MAb’s or poly-HI-AB’s